Mathematical models and computational approaches in CAR-T therapeutics
- PMID: 40821789
- PMCID: PMC12354594
- DOI: 10.3389/fimmu.2025.1581210
Mathematical models and computational approaches in CAR-T therapeutics
Erratum in
-
Correction: Mathematical models and computational approaches in CAR-T therapeutics.Front Immunol. 2025 Sep 8;16:1694607. doi: 10.3389/fimmu.2025.1694607. eCollection 2025. Front Immunol. 2025. PMID: 40990015 Free PMC article.
Abstract
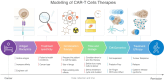
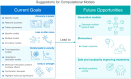
Background: The field of synthetic biology aims to engineer living organisms for specific therapeutic applications, with CAR-T cell therapy emerging as a groundbreaking approach in cancer treatment due to its potential for flexibility, specificity, predictability, and controllability. CAR-T cell therapies involve the genetic modification of T cells to target tumor-specific antigens. However, challenges persist because the limited spatio-temporal resolution in current models hinders the therapy's safety, cost-effectiveness, and overall potential, particularly for solid tumors.
Main body: This manuscript explores how mathematical models and computational techniques can enhance CAR-T therapy design and predict therapeutic outcomes, focusing on critical factors such as antigen receptor functionality, treatment efficacy, and potential adverse effects. We examine CAR-T cell dynamics and the impact of antigen binding, addressing strategies to overcome antigen escape, cytokine release syndrome, and relapse.
Conclusion: We propose a comprehensive framework for using these models to advance CAR-T cell therapy, bridging the gap between existing therapeutic methods and the full potential of CAR-T engineering and its clinical application.
Keywords: CAR-T cells; T cell engineering; biological system modeling; computational immunotherapy; mathematical modeling; synthetic biology; therapeutic optimization.
Copyright © 2025 Putignano, Ruipérez-Campillo, Yuan, Millet and Guerrero-Aspizua.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

