Choline in immunity: a key regulator of immune cell activation and function
- PMID: 40821837
- PMCID: PMC12353711
- DOI: 10.3389/fimmu.2025.1617077
Choline in immunity: a key regulator of immune cell activation and function
Abstract
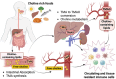
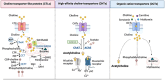
Nutrient availability is a strong determinant of cell function. Immune cells, which must rapidly activate transcriptional, proteomic, and metabolic programs to fulfill their functional roles, depend on nutrient supply to generate the building blocks needed for the production of immune effectors. While glucose, glutamine, and amino acids are well-recognized as critical energy sources and carbon donors during immune activation, the contribution of choline, a vitamin-like metabolite, has been overlooked. Once taken up by cells, choline plays a vital role in several biological processes. It is a precursor for phosphatidylcholine, the primary phospholipid in cellular membranes, and is also essential for synthesizing the neurotransmitter acetylcholine. Additionally, when directed toward mitochondria and betaine synthesis, choline serves as a methyl donor for histone and protein methylation, key processes that regulate gene expression and cellular activity. In this review, we examine the latest research on how immune cells utilize and metabolize choline, as well as its broader implications for immune-related disorders and overall human health. We also discuss recent and ongoing clinical studies investigating the effects of choline supplementation and the potential use of choline-derived metabolites as biomarkers for therapy response.
Keywords: acetylcholine (ACh); choline; choline kinase (ChoK); immune cells; immunity; inflammation; phosphatidylcholine (PC); phosphocholine.
Copyright © 2025 Maia, Fung and Sanchez-Lopez.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
-
T-bet expressing Tr1 cells driven by dietary signals dominate the small intestinal immune landscape.bioRxiv [Preprint]. 2025 Jul 4:2025.06.30.662190. doi: 10.1101/2025.06.30.662190. bioRxiv. 2025. PMID: 40747421 Free PMC article. Preprint.
References
-
- Jieru P, Zhang S, Cai L, Long W, Wang Y, Zhang L, et al. Dietary choline intake and health outcomes in U.S. adults: exploring the impact on cardiovascular disease, cancer prevalence, and all-cause mortality. J Health Popul Nutr. (2024) 43:59. doi: 10.1186/s41043-024-00528-0, PMID: - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

