CRIF1 gene therapy ameliorates inflammatory bowel disease by suppressing TH17 cells and fibrosis through mitochondrial function regulation
- PMID: 40821844
- PMCID: PMC12350146
- DOI: 10.3389/fimmu.2025.1618012
CRIF1 gene therapy ameliorates inflammatory bowel disease by suppressing TH17 cells and fibrosis through mitochondrial function regulation
Abstract
Background: CR6-interacting factor 1 (CRIF1) is a nuclear transcriptional regulator and a mitochondrial inner membrane protein. Although serious modifications of the tissue architecture of the small intestine have been reported in CRIF1-deficient mice, how this may affect the development of inflammatory bowel disease (IBD) remains unclear. We investigated the effects of CRIF1 on mice with colitis.
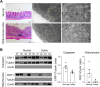
Methods: In DSS-induced colitis mice administered p3XFLAG-CMV-10-CRIF1, clinical symptoms were evaluated. Mitochondrial morphology in the intestinal tissues of colitis mice and UC patients was observed by electron microscopy. Level of CRIF1 in the splenic mitochondria of colitis mice or human PBMCs were investigated by western blot or real-time PCR, and the amount of IL-17 in the supernatant of healthy PBMCs co-cultured with CRIF1-overexpressing mitochondria was investigated by ELISA.
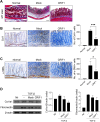
Results: Overexpression of CRIF1 attenuated the severity of colitis, alleviated weight loss, and intestinal shortening. Moreover, overexpression of CRIF1 significantly reduced the levels of proinflammatory and necroptosis-related factors in colon and inhibited intestinal fibrosis. The intestines of these mice showed a reduced level of CRIF1 and altered mitochondrial morphology. Transplantation of CRIF1-overexpressed mitochondria into mice with colitis alleviated disease severity. Patients with ulcerative colitis exhibited decreased CRIF1 levels with dysfunctional mitochondria in inflamed colonic tissue. CRIF1-overexpressing mitochondria inhibited IL-17 production in PBMCs from healthy control.
Conclusion: Our findings demonstrate that CRIF1 alleviates IBD by suppressing inflammation and fibrosis by improving mitochondrial function. Improving mitochondrial function through CRIF1 may be a potential therapeutic strategy for IBD.
Keywords: CRIF1; fibrosis; inflammatory bowel disease; inflammatory cytokine; mitochondria.
Copyright © 2025 Park, Kang, Jeong, Yang, Choi, Cho, Kim, Lee and Cho.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures





Similar articles
-
Intestinal inflammation and microbiota modulation impact cochlear function: emerging insights in gut-ear axis.Cell Commun Signal. 2025 Jul 26;23(1):357. doi: 10.1186/s12964-025-02338-1. Cell Commun Signal. 2025. PMID: 40713718 Free PMC article.
-
Gegen Qinlian decoction ameliorates TNBS-induced ulcerative colitis by regulating Th2/Th1 and Tregs/Th17 cells balance, inhibiting NLRP3 inflammasome activation and reshaping gut microbiota.J Ethnopharmacol. 2024 Jun 28;328:117956. doi: 10.1016/j.jep.2024.117956. Epub 2024 Feb 29. J Ethnopharmacol. 2024. PMID: 38428658
-
Parishin from Gastrodia elata ameliorates DSS induced colitis and anxiety-like behavior in mice by regulating intestinal barrier function and microviota-gut-brain axis.Phytomedicine. 2025 Sep;145:157019. doi: 10.1016/j.phymed.2025.157019. Epub 2025 Jun 22. Phytomedicine. 2025. PMID: 40582206
-
Strategies for detecting colon cancer and/or dysplasia in patients with inflammatory bowel disease.Cochrane Database Syst Rev. 2006 Apr 19;(2):CD000279. doi: 10.1002/14651858.CD000279.pub3. Cochrane Database Syst Rev. 2006. Update in: Cochrane Database Syst Rev. 2017 Sep 18;9:CD000279. doi: 10.1002/14651858.CD000279.pub4. PMID: 16625534 Updated.
-
Patient education interventions for the management of inflammatory bowel disease.Cochrane Database Syst Rev. 2023 May 4;5(5):CD013854. doi: 10.1002/14651858.CD013854.pub2. Cochrane Database Syst Rev. 2023. PMID: 37172140 Free PMC article.
References
-
- Collaborators GBDIBD . The global, regional, and national burden of inflammatory bowel disease in 195 countries and territories, 1990-2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol Hepatol. (2020) 5:17–30. doi: 10.1016/S2468-1253(19)30333-4, PMID: - DOI - PMC - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical

