Anisotropic liquid crystalline hydrogels direct 2D and 3D myoblast alignment
- PMID: 40821878
- PMCID: PMC12356246
- DOI: 10.1002/adfm.202518226
Anisotropic liquid crystalline hydrogels direct 2D and 3D myoblast alignment
Abstract
Tissue development and regeneration are governed by processes that span subcellular signaling, cell-cell interactions, and the integrated mechanical properties of cellular collectives with their extracellular matrix. Synthetic biomaterials that can emulate the hierarchical structure and supracellular mechanics of living systems are paramount to the realization of regenerative medicine. Recent reports detail directed cell alignment on mechanically anisotropic but stiff liquid crystalline polymer networks (LCNs). While compelling, the potential implementation of these materials as tissue engineering scaffolds may be hindered by the orders of magnitude larger stiffness than most soft tissue. Accordingly, this report prepares liquid crystalline hydrogels (LCHs) that synergize the anisotropic mechanical properties intrinsic to LCNs with the cytocompatibility and soft mechanics of PEG hydrogels. LCH are prepared via sequential oligomerization and photopolymerization reactions between liquid crystalline (LC) monomers and poly(ethylene glycol) (PEG)-dithiol. Despite their low liquid crystalline content, swollen LCH oligomers are amenable to rheological alignment via direct ink write 3D printing. Mechanically anisotropic LCHs support C2C12 myoblast culture on their surface and direct their alignment in the stiffest direction. Further, C2C12s can be encapsulated within LCH oligomers and 3D-printed, whereby mechanical anisotropy of the LCH directs myoblast polarization in 3D.
Keywords: 3D bioprinting; anisotropic hydrogel; cellular alignment; liquid crystalline; tissue engineering.
Conflict of interest statement
Conflicts of Interest The authors declare no conflict of interest.
Figures
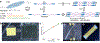

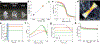


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Anisotropic hydrogel degradation enhances 3D collective mesenchymal stromal cell alignment, mechanotransduction and osteogenic differentiation.Acta Biomater. 2025 Jul 29:S1742-7061(25)00569-0. doi: 10.1016/j.actbio.2025.07.063. Online ahead of print. Acta Biomater. 2025. PMID: 40744131
-
Comparative Analysis of Hydrogels From Porcine Extracellular Matrix for 3D Bioprinting of Adipose Tissue.J Biomed Mater Res A. 2025 Apr;113(4):e37832. doi: 10.1002/jbm.a.37832. J Biomed Mater Res A. 2025. PMID: 40165526
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Management of urinary stones by experts in stone disease (ESD 2025).Arch Ital Urol Androl. 2025 Jun 30;97(2):14085. doi: 10.4081/aiua.2025.14085. Epub 2025 Jun 30. Arch Ital Urol Androl. 2025. PMID: 40583613 Review.
References
Grants and funding
LinkOut - more resources
Full Text Sources
