Ferroptosis as a therapeutic target in glioblastoma: Mechanisms and emerging strategies
- PMID: 40822033
- PMCID: PMC12356316
- DOI: 10.1016/j.omtn.2025.102649
Ferroptosis as a therapeutic target in glioblastoma: Mechanisms and emerging strategies
Abstract
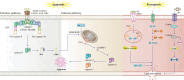
Glioblastoma multiforme (GBM) is the most prevalent malignant brain tumor. Treating this type of cancer is challenging due to its high heterogeneity, rapid cell growth, and highly malignant nature, which results in a poor prognosis. A key feature of GBM's malignancy is that it resists drug treatments and evades cell death mechanisms. Ferroptosis is a promising therapeutic avenue for combating drug-resistant cancers because it is a recently discovered mechanism of programmed cell death that oxidizes membrane lipids and is triggered by an accumulation of reactive oxygen species. Recent findings suggest that ferroptosis is an innovative path for improving human GBM therapy. More exploration of the regulatory pathways and interactions of ferroptosis is essential to developing effective therapeutic strategies for this aggressive type of cancer. Inducing ferroptosis or integrating it with current treatments may present an opportunity to improve outcomes in GBM patients. This review investigates the role of ferroptosis in GBM and identifies its important molecular mediators. It also explores promising therapeutic strategies that target ferroptosis as a novel approach for GBM treatment.
Keywords: MT: Oligonucleotides: Therapies and Applications; ferroptosis; ferroptosis mediator; glioblastoma multiforme; glutathione depletion; iron metabolism; lipid peroxidation; targeting treatment.
© 2025 The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Ioning out glioblastoma: ferroptosis mechanisms and therapeutic frontiers.Cell Death Discov. 2025 Aug 26;11(1):407. doi: 10.1038/s41420-025-02711-6. Cell Death Discov. 2025. PMID: 40858533 Free PMC article. Review.
-
Pediatric Diffuse High-Grade Gliomas: A Comprehensive Review Of Ad-vanced Methods Of Diagnosis And Treatment.Curr Cancer Drug Targets. 2025 Jun 30. doi: 10.2174/0115680096365252250618115641. Online ahead of print. Curr Cancer Drug Targets. 2025. PMID: 40598730
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Ginsenoside F2-modified liposomes delivering FTY720 enhance glioblastoma targeting and antitumor activity via ferroptosis.Phytomedicine. 2025 Aug;144:156917. doi: 10.1016/j.phymed.2025.156917. Epub 2025 May 30. Phytomedicine. 2025. PMID: 40480023
References
Publication types
LinkOut - more resources
Full Text Sources

