First Report of Hematopoietic Stem Cell Transplantation for Children Diagnosed with Wiskott-Aldrich Syndrome in Vietnam
- PMID: 40822038
- PMCID: PMC12357556
- DOI: 10.2147/JBM.S528827
First Report of Hematopoietic Stem Cell Transplantation for Children Diagnosed with Wiskott-Aldrich Syndrome in Vietnam
Abstract
Background: Awareness of inborn error immunity, such as Wiskott-Aldrich syndrome (WAS), is still lacking in Vietnam. The shortage of clinical immunologists and transplantation teams lead to poor prognosis for patients.
Objective: Describe initial data about hematopoietic stem cell transplantation (HSCT) for WAS.
Methods: Retrospective analyzing 15 procedures on 13 patients at the Vietnam National Children's Hospital from 2020 to 2024.
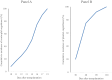
Results: The median age at HSCT was 34 months (range: 17-86). Of the patients, 73.3% received myeloablative conditioning based on busulfan, while 26.7% underwent reduced-intensity conditioning. Donor sources included matched sibling donors (MSD, 20.0%), unrelated cord blood (UCB, 33.3%), phenotypically identical family donor (MFD, 6.7%), and mismatched related donors (MMRD, 40.0%). The median stem cell dose was 4.9 × 10^6/kg of the recipient's body weight (range: 0.33 to 10.4 × 10^6/kg). Neutrophil and platelet engraftment occurred at a median of 14 days (range: 10-19) and 48 days (range: 14-143), respectively. By day +30, 73.3% of patients achieved full donor chimerism. One patient experienced graft failure, and another faced graft rejection two months post-transplant, both of whom underwent a second transplant with different donors. The overall survival rate was 92.3% with a median follow-up of 23 months (range: 6-53), with one patient died from chronic graft-versus-host disease (cGVHD). All surviving patients achieved normalization of platelet counts.
Conclusion: HSCT offers significant benefits to WAS patients, achieving an excellent overall survival rate.
Keywords: Wiskott-Aldrich syndrome; hematopoietic stem cell transplantation; inborn error immunity; pediatric; thrombocytopenia.
© 2025 Nguyen-Ngoc-Quynh et al.
Conflict of interest statement
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Figures
Similar articles
-
First-line allogeneic hematopoietic stem cell transplantation of HLA-matched sibling donors compared with first-line ciclosporin and/or antithymocyte or antilymphocyte globulin for acquired severe aplastic anemia.Cochrane Database Syst Rev. 2013 Jul 23;2013(7):CD006407. doi: 10.1002/14651858.CD006407.pub2. Cochrane Database Syst Rev. 2013. PMID: 23881658 Free PMC article.
-
Bone marrow versus peripheral blood allogeneic haematopoietic stem cell transplantation for haematological malignancies in adults.Cochrane Database Syst Rev. 2014 Apr 20;2014(4):CD010189. doi: 10.1002/14651858.CD010189.pub2. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2024 Nov 7;11:CD010189. doi: 10.1002/14651858.CD010189.pub3. PMID: 24748537 Free PMC article. Updated.
-
Allogeneic Hematopoietic Cell Transplantation for Juvenile Myelomonocytic Leukemia with a Busulfan, Fludarabine, and Melphalan Regimen: JPLSG JMML-11.Transplant Cell Ther. 2024 Jan;30(1):105.e1-105.e10. doi: 10.1016/j.jtct.2023.10.002. Epub 2023 Oct 7. Transplant Cell Ther. 2024. PMID: 37806448
-
Upfront Haploidentical Hematopoietic Cell Transplantation Using αβ+ T Cell-Depleted Peripheral Blood Stem Cells for Pediatric Patients with Acquired Severe Aplastic Anemia.Transplant Cell Ther. 2025 Jun 10:S2666-6367(25)01255-2. doi: 10.1016/j.jtct.2025.06.012. Online ahead of print. Transplant Cell Ther. 2025. PMID: 40505813
-
Pilot Study of Donor-Engrafted Clonal Hematopoiesis Evolution and Clinical Outcomes in Allogeneic Hematopoietic Cell Transplantation Recipients Using a National Registry.Transplant Cell Ther. 2023 Oct;29(10):640.e1-640.e8. doi: 10.1016/j.jtct.2023.07.021. Epub 2023 Jul 28. Transplant Cell Ther. 2023. PMID: 37517612 Free PMC article.
References
LinkOut - more resources
Full Text Sources