ThDP-dependent enzyme catalyzed oxidation of aldehydes
- PMID: 40822103
- PMCID: PMC12352684
- DOI: 10.1039/d5sc03250d
ThDP-dependent enzyme catalyzed oxidation of aldehydes
Abstract
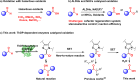
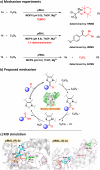
Natural thiamine diphosphate (ThDP)-dependent enzymes are frequently utilized to catalyze the decarboxylation of β-keto acids and the benzoin condensation of aldehydes. Herein, we present a ThDP-dependent enzymatic oxidation of aldehydes mediated by sequential single electron transfer (SET) processes, utilizing hexachloroethane (C2Cl6) as the oxidant. The reaction exhibits high efficiency (yield up to 99% and turnover number up to 2000) and achieves effective stereoselective control for dynamic kinetic resolutions (e.e. up to 99%). This study uncovers a previously undiscovered capability of ThDP-dependent enzymes, thus broadening the functional repertoire of this enzyme class.
This journal is © The Royal Society of Chemistry.
Conflict of interest statement
There are no conflicts to declare.
Figures

Similar articles
-
Trihydroxybenzaldoximes are Redox Cycling Inhibitors of ThDP-Dependent DXP Synthase.ACS Chem Biol. 2025 Jun 20;20(6):1195-1211. doi: 10.1021/acschembio.5c00025. Epub 2025 May 18. ACS Chem Biol. 2025. PMID: 40383931
-
Trihydroxybenzaldoximes are redox cycling inhibitors of ThDP-dependent DXP synthase.bioRxiv [Preprint]. 2025 Mar 5:2025.03.03.641193. doi: 10.1101/2025.03.03.641193. bioRxiv. 2025. Update in: ACS Chem Biol. 2025 Jun 20;20(6):1195-1211. doi: 10.1021/acschembio.5c00025. PMID: 40093103 Free PMC article. Updated. Preprint.
-
Diversity of ThDP-Dependent Enzymes Forming Chiral Tertiary Alcohols.Chembiochem. 2025 Jul 11;26(13):e202500200. doi: 10.1002/cbic.202500200. Epub 2025 May 27. Chembiochem. 2025. PMID: 40228089 Free PMC article. Review.
-
Combined Administration of Metformin and Amprolium to Rats Affects Metabolism of Free Amino Acids in the Brain, Altering Behavior, and Heart Rate.Biochemistry (Mosc). 2024 Oct;89(10):1692-1710. doi: 10.1134/S0006297924100043. Biochemistry (Mosc). 2024. PMID: 39523110
-
Drugs for preventing postoperative nausea and vomiting in adults after general anaesthesia: a network meta-analysis.Cochrane Database Syst Rev. 2020 Oct 19;10(10):CD012859. doi: 10.1002/14651858.CD012859.pub2. Cochrane Database Syst Rev. 2020. PMID: 33075160 Free PMC article.
References
-
- March J., Advanced Organic Chemistry: Reactions, Mechanisms and Structure, 4th edn, Wiley, New York, 1992
- Collins T. J. Designing Ligands for Oxidizing Complexes. Acc. Chem. Res. 1994;27:279–285.
- Smith M. B. and March J., Advanced Organic Chemistry: Reactions, Mechanisms and Structure, 6th edn, Wiley, Hoboken, 2007
- Tojo G. and Fernández M., Oxidation of Primary Alcohols to Carboxylic Acids, Springer, New York, 2010
- Bruice P. Y., Organic Chemistry, Prentice Hall, Englewood Cliffs, NJ, 2012
-
- Thottathil J. K. Moniot J. L. Mueller R. H. Wong M. K. Y. Kissick T. P. J. Org. Chem. 1986;51:3140–3143.
-
- Mahmood A. Robinson A. Powell L. Org. Process Res. Dev. 1999;3:363–364.
-
- Hunsen M. Synthesis. 2005;15:2487–2490.
LinkOut - more resources
Full Text Sources

