MLR Corresponds to the Functional Status of Monocytes in Chronic Lymphocytic Leukemia
- PMID: 40822140
- PMCID: PMC12356674
- DOI: 10.1155/ijin/4443773
MLR Corresponds to the Functional Status of Monocytes in Chronic Lymphocytic Leukemia
Abstract
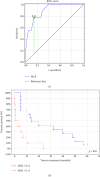
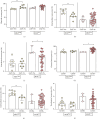
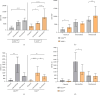
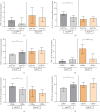
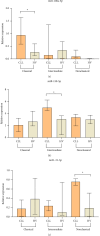
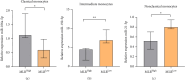
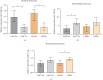
Background: The role of the inflammatory microenvironment in initiating and progressing chronic lymphocytic leukemia (CLL) is still not clarified. To date, it has been shown that the only way to reflect inflammation in the systemic circulation is to assess inflammatory markers in peripheral blood. However, in the age of modern technology, a more detailed analysis of inflammatory cells circulating in the blood of CLL patients would be useful. Objectives: The study aimed to evaluate the relationship between one of the hematological inflammatory indexes-the monocyte/lymphocyte ratio (MLR) and the risk of CLL progression associated with disease activity. In addition, we wanted to analyze whether the MLR parameter in CLL could suggest the functional immune status of circulating main monocyte subsets. Methods: The study included peripheral blood samples from 54 untreated, newly diagnosed CLL patients and 20 healthy volunteers (HVs). Immunological characterization of monocyte subpopulations included their detailed assessment by multiparametric flow cytometry, including evaluation of surface markers and intracellular expression of cytokines. In addition, the relative expression of selected microRNA (miR-21-3p, miR-150-5p, miR-106a-5p) was determined in FACS-sorted monocyte subsets. Results: In our study, CLL patients had significantly lower values of MLR parameters compared to HVs (p < 0.0001). However, the value of MLR was higher in CLL patients with negative clinical and laboratory prognostic factors, i.e., increased percentage of CD5+/CD19+ cells with ZAP-70 and CD38 expression. We noticed that the percentage of intermediate monocytes is significantly higher, but classical and nonclassical ones are significantly lower in MLR-high compared to MLR-low CLL patients. Moreover, among the monocyte subsets circulating in the blood of MLR-high, ZAP-70+, and CD38+, CLL patients' intermediate monocytes were characterised by increased intracellular expression of IL-10 and decreased miR-150-5p relative expression compared to intermediate monocytes in the MLR-low, ZAP-70-, and CD38- groups, suggesting a potential link between hematological inflammatory index and the formation of intermediate monocytes that promote CLL burden. Conclusions: The MLR index may serve not only as a marker of CLL activity, but also indirectly indicate changes in the phenotype and function of monocyte subpopulations present in the blood microenvironment. Moreover, the MLR-high parameter seems to correspond to an increase in the percentage of intermediate monocytes with anti-inflammatory properties, which may potentially promote disease progression and worsen its prognosis.
Copyright © 2025 Wioleta Grzegorzewska et al. International Journal of Inflammation published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
[Volume and health outcomes: evidence from systematic reviews and from evaluation of Italian hospital data].Epidemiol Prev. 2013 Mar-Jun;37(2-3 Suppl 2):1-100. Epidemiol Prev. 2013. PMID: 23851286 Italian.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2021 Apr 19;4(4):CD011535. doi: 10.1002/14651858.CD011535.pub4. Cochrane Database Syst Rev. 2021. Update in: Cochrane Database Syst Rev. 2022 May 23;5:CD011535. doi: 10.1002/14651858.CD011535.pub5. PMID: 33871055 Free PMC article. Updated.
References
-
- Vilchis-Ordoñez A., Ramírez-Ramírez D., Pelayo R. The Triad Inflammation-Microenvironment-Tumor Initiating Cells in Leukemia Progression. Current Opinion in Physiology . 2021;19:211–218. doi: 10.1016/j.cophys.2020.10.010. - DOI
-
- Mukkamalla S. K. R., Taneja A., Malipeddi D., et al. Statpearls . StatPearls Publishing; 2023. Chronic Lymphocytic Leukemia. https://europepmc.org/books/NBK470433 . - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

