Physiologically-Based Pharmacokinetics and Empirical Pharmacodynamic Modeling for Pediatric Henagliflozin Dosing: Clinical Insights for Chinese Patients
- PMID: 40822299
- PMCID: PMC12352996
- DOI: 10.1155/pedi/8857248
Physiologically-Based Pharmacokinetics and Empirical Pharmacodynamic Modeling for Pediatric Henagliflozin Dosing: Clinical Insights for Chinese Patients
Abstract
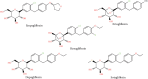
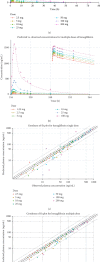
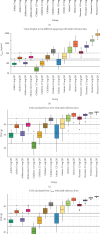
Objective: This study aimed to present a quantitative modeling and simulation approach for oral henagliflozin, a selective sodium-glucose cotransporter 2 (SGLT2) inhibitor primarily metabolized by uridine diphosphate-glucuronosyltransferase (UGT) enzymes. Methods: A physiologically-based pharmacokinetic (PBPK) model for henagliflozin was developed using in vitro metabolism and clinical pharmacokinetic (PK) data, with validation across multiple contexts, including healthy adults, and hepatic impairment populations. Additionally, empirical pharmacodynamic (PD) modeling was employed to optimize pediatric dosing based on exposure-response relationships for urinary glucose excretion (UGE). Predicting henagliflozin exposure in pediatric patients poses challenges due to UGT enzyme ontogeny and the scarcity of clinical PK data in younger age groups. Using twofold acceptance criteria, model-predicted and observed drug exposures and PK parameters (area under the curve and peak concentration) were compared in diverse scenarios, including monotherapy in healthy adults (single/multiple doses), hepatic impairment, and extrapolation to pediatric age groups. Results: The PBPK model accurately captured observed exposures within a twofold range in both adults and adolescents, supporting the model's predictive utility. The verified PBPK and empirical PD models informed dosing recommendations in pediatric populations aged 1 month to 18 years, achieving henagliflozin exposures comparable to those in adult patients receiving a 5-10 mg dose. Conclusion: This study shows that PBPK and PD modeling effectively guide pediatric dosing of henagliflozin, reducing trial reliance and supporting real-world validation.
Keywords: Chinese population; henagliflozin; pediatric dosing; pharmacodynamic (PD) modeling; physiologically-based pharmacokinetic (PBPK) modeling.
Copyright © 2025 Xinyue Zhang et al. Pediatric Diabetes published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures



References
-
- Wang Y., Jiang C., Dong X., et al. Combination of Retagliptin and Henagliflozin as Add-on Therapy to Metformin in Patients With Type 2 diabetes Inadequately Controlled With Metformin: A Multicentre, Randomized, Double-Blind, Active-Controlled, Phase 3 Trial. Diabetes, Obesity and Metabolism . 2024;26(7):2774–2786. doi: 10.1111/dom.15596. - DOI - PubMed
-
- Weng J., Zeng L., Zhang Y., et al. Henagliflozin as Add-on Therapy to Metformin in Patients With Type 2 Diabetes Inadequately Controlled With Metformin: A Multicentre, Randomized, Double-Blind, Placebo-Controlled, Phase 3 Trial. Diabetes, Obesity and Metabolism . 2021;23(8):1754–1764. doi: 10.1111/dom.14389. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources

