The functional roles of deoxyelephantopin potential target circTNPO3 in regulating pancreatic cancer malignant phenotype and gemcitabine chemoresistance via miR-188-5p/CDCA3/TRAF2-mediated remodeling of NF-κB signaling pathway
- PMID: 40822457
- PMCID: PMC12350284
- DOI: 10.3389/fphar.2025.1613560
The functional roles of deoxyelephantopin potential target circTNPO3 in regulating pancreatic cancer malignant phenotype and gemcitabine chemoresistance via miR-188-5p/CDCA3/TRAF2-mediated remodeling of NF-κB signaling pathway
Abstract
Background: Pancreatic cancer (PC) has been one of the most severe digestive system malignant tumor with poor prognosis that threatens human health. Chemotherapy is essential for patients with advanced PC, but unfortunately the curative effect is limited by chemoresistance. CircTNPO3, a recently discovered circular RNA (circRNA), has been indicated to be associated with multi-types of tumors. However, the function and mechanism of circTNPO3 in regulating PC malignant phenotype and chemoresistance still remain obscure.
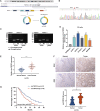
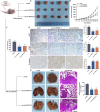
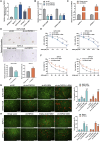
Methods: qRT-PCR and ISH were used to analyze circTNPO3 expression in PC cells and pathological specimens. The subcellular localization of circTNPO3 was visualized through nucleoplasmic RNA separation and FISH assays. The effect of cicTNPO3 on PC cell proliferation, migration and invasion was assessed using EdU, colony formation, wound healing and Transwell assays respectively. Cell apoptosis was detected using ELISA, AO/EB, Hoechst 33342 and flow cytometry assays. The binding potential between circTNPO3, miR-188-5p and CDCA3 was verified by Ago2-RIP, RNA pull down and dual-luciferase reporter assays. The relationship between CDCA3, TRAF2 and NF-κB-p65 was analyzed using Pearson correlation, and the expression was detected using immunoblotting. The nucleus translocation of p65 was evaluated using IF assay. The effect of circTNPO3 on PC growth and metastasis was analyzed using subcutaneous and lung metastatic tumor models in vitro. Deoxyelephantopin, a small molecule extract from traditional Chinese medicine, was applied to evaluate the potential of circTNPO3 as therapeutic target.
Results: CircTNPO3 was aberrantly highly expressed in PC cells and tissues, and negatively associated with patient prognosis and gemcitabine chemotherapy sensitivity. Functionally, silencing circTNPO3 attenuated the malignant phenotypes and chemoresistance of PC in vitro and in vivo, conversely, facilitated by circTNPO3 overexpression. Mechanically, cytoplasmic circTNPO3 functioned as a sponge of miR-188-5p, and partially alleviated the effect of miR-188-5p on downstream molecules, which further upregulate the CDCA3 and TRAF2 expression and NF-κB activity, finally promoted PC progression and chemoresistance. More innovatively, the potential of circTNPO3 as a novel diagnostic biomarker and therapeutic target for PC was primarily validated in present study.
Conclusion: CircTNPO3 acted as an oncogenic and chemoresistant gene in PC, mechanically through targeting miR-188-5p and regulating CDCA3, TRAF2 and NF-κB signaling pathway.
Keywords: NF-κB signaling pathway; chemoresistance; circTNPO3; deoxyelephantopin; gemcitabine; pancreatic cancer; stemness.
Copyright © 2025 Ji, Liu, Zhang, Hou, Feng, Xing, Guan, Cui, Xu and Tan.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Dahuang Zhechong Pill Combined with TNS4 Silencing Inhibits the NF-Κb/VEGF Pathway To Slow Down The Progression Of Pancreatic Cancer.Recent Pat Anticancer Drug Discov. 2025 Jul 3. doi: 10.2174/0115748928379439250621171215. Online ahead of print. Recent Pat Anticancer Drug Discov. 2025. PMID: 40619656
-
Mesenchymal stem cell-derived lncRNAs NKILA contributes to stemness and chemoresistance by fatty acid oxidation in gastric cancer via miR-485-5p/STAT3.World J Gastrointest Oncol. 2025 Aug 15;17(8):105006. doi: 10.4251/wjgo.v17.i8.105006. World J Gastrointest Oncol. 2025. PMID: 40837764 Free PMC article.
-
CircASH1L inhibits ferroptosis and enhances cisplatin resistance by sponging miR-515-5p to regulate cell cycle-related CDCA7/RRM2 in ovarian cancer cells.Front Pharmacol. 2025 Jun 24;16:1563869. doi: 10.3389/fphar.2025.1563869. eCollection 2025. Front Pharmacol. 2025. PMID: 40630134 Free PMC article.
-
A rapid and systematic review of the clinical effectiveness and cost-effectiveness of paclitaxel, docetaxel, gemcitabine and vinorelbine in non-small-cell lung cancer.Health Technol Assess. 2001;5(32):1-195. doi: 10.3310/hta5320. Health Technol Assess. 2001. PMID: 12065068
-
Signs and symptoms to determine if a patient presenting in primary care or hospital outpatient settings has COVID-19.Cochrane Database Syst Rev. 2022 May 20;5(5):CD013665. doi: 10.1002/14651858.CD013665.pub3. Cochrane Database Syst Rev. 2022. PMID: 35593186 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

