Forkhead box O proteins in chondrocyte aging and diseases
- PMID: 40822516
- PMCID: PMC12357264
- DOI: 10.1016/j.jot.2025.07.011
Forkhead box O proteins in chondrocyte aging and diseases
Abstract
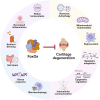
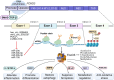
As people age, the progressive loss of cartilage integrity occurs, accompanied by a decline in the capacity to repair. This results in decreased resilience and increased susceptibility of cartilage to various physiological stressors, which raises the risk of developing osteoarthritis (OA). Therefore, restoring the regenerative capacity of chondrocytes and slowing down the aging process could be promising therapeutic strategies to mitigate or even reverse age-related joint diseases. Forkhead box class O (FoxO) proteins are a family of transcription factors that play a crucial role in various cellular processes linked to aging. Their significant functions in cell cycle regulation, apoptosis, and resistance to oxidative stress highlight their importance in maintaining cellular homeostasis and promoting longevity. In this review, we introduce the structures and functions of FoxO proteins in chondrocytes, focusing on their spatiotemporal regulation of epigenetics during chondrocyte differentiation stages in different layers. The critical roles of FoxO proteins in maintaining chondrocyte homeostasis are summarized, alongside a discussion of how FoxO dysfunction contributes to aging and OA. Furthermore, therapeutic strategies targeting FoxO proteins to mitigate aging-related cartilage degradation and decelerate OA progression are explored. Finally, potential directions for future research are proposed to deepen the current understanding of FoxO proteins.
The translational potential of this article: FoxO transcription factors, especially FoxO1 and FoxO3, are promising therapeutic targets for promoting longevity, stimulating cartilage regeneration, and treating aging-related diseases like OA.
Keywords: Aging; Cartilage regeneration; Chondrocyte; Forkhead box class O; FoxO; Osteoarthritis; Rejuvenation.
© 2025 The Authors.
Conflict of interest statement
All authors declare that there are no conflicts of interest.
Figures


References
-
- Global incidence, prevalence, years lived with disability (ylds), disability-adjusted life-years (dalys), and healthy life expectancy (hale) for 371 diseases and injuries in 204 countries and territories and 811 subnational locations, 1990-2021: a systematic analysis for the global burden of disease study 2021. Lancet. 2024;403(10440):2133–2161. - PMC - PubMed
-
- Weng Q., Chen Q., Jiang T., Zhang Y., Zhang W., Doherty M., et al. Global burden of early-onset osteoarthritis, 1990-2019: results from the global burden of disease study 2019. Ann Rheum Dis. 2024;83(7):915–925. - PubMed
-
- Tang S., Zhang C., Oo W.M., Fu K., Risberg M.A., Bierma-Zeinstra S.M., et al. Osteoarthritis. Nat Rev Dis Primers. 2025;11(1):10. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

