Narirutin mitigates inflammatory arthritis and osteoporosis through modulating macrophage phenotype and osteoclastogenesis
- PMID: 40822517
- PMCID: PMC12356004
- DOI: 10.1016/j.jot.2025.07.008
Narirutin mitigates inflammatory arthritis and osteoporosis through modulating macrophage phenotype and osteoclastogenesis
Abstract
Background: Inflammatory arthritis (IA), exemplified by rheumatoid arthritis (RA), represents a prevalent autoimmune-driven inflammatory bone disorder hallmarked by chronic synovitis and progressive bone erosion, culminating in joint dysfunction and systemic osteoporosis. Narirutin (NRT), a flavonoid glycoside derived from citrus plants, is renowned for its multifaceted bioactivities, including antioxidant, immunomodulatory, and cardioprotective properties. Despite these attributes, the role of NRT in mitigating macrophage-mediated pro-inflammatory activation and osteoclastogenesis within the context of inflammatory arthritis and osteoporosis remains insufficiently elucidated. This study aimed to evaluate the therapeutic potential of NRT in the context of inflammatory arthritis and osteoporosis.
Methods: The phenotypic modulation of macrophages and the osteoclastogenic effects of NRT were evaluated using RAW264.7, THP-1 and bone marrow-derived macrophages (BMMs) in vitro. A classical collagen-induced arthritis (CIA) model was established to investigate the therapeutic effects of NRT administration on inflammatory arthritis and osteoporosis. Macrophage phenotypes and the expression of inflammatory mediators were analyzed in vitro and vivo, respectively. High-throughput RNA sequencing and bioinformatics analyses were employed to identify key downstream signaling pathways, which were further validated. Histological staining, micro-CT, and immunehistofluorescence staining were utilized to assess the in vivo amelioration of inflammation and bone destruction. Visceral toxicity was also assessed in vivo.
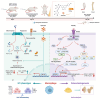
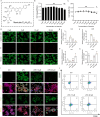
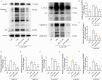
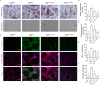
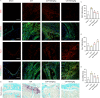
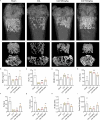
Results: NRT markedly inhibited lipopolysaccharide (LPS)-induced macrophage polarization towards the pro-inflammatory M1 phenotype (CD86+), while promoting a shift towards the anti-inflammatory M2 phenotype (CD206+). This was accompanied by a suppression of pro-inflammatory cytokines, including iNOS, TNF, IL-1β, and IL-6, and an upregulation of immunosuppressive mediators such as IL-10 and Arg-1. RNA sequencing revealed that NRT attenuates the activation of the NOD-like receptor signaling pathway and downstream inflammasome activation. Additionally, osteoclast differentiation was also significantly inhibited, as evidenced by the suppression of NF-κB and MAPK signaling pathways. In vivo studies demonstrated that NRT substantially alleviates the severity of inflammatory arthritis and mitigates systemic osteoporosis.
Conclusion: These findings demonstrated that NRT mitigates inflammatory arthritis and osteoporosis through modulating macrophage phenotype and osteoclastogenesis via NOD-like receptor signaling pathway induced inflammasome activation and NF-κB and MAPK signaling pathways, respectively.
The translational potential of this article: These findings highlight the potential of targeting macrophage pro-inflammatory M1 phenotype in and osteoclastogenesis as an effective strategy for inflammatory arthritis and systemic osteoporosis, and positioning NRT may serve as a promising therapeutic drug candidate.
Keywords: Inflammatory arthritis; Macrophage polarization; Narirutin; Osteoclast; Osteoimmunomodulatory; Osteoporosis.
© 2025 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships.
Figures






References
-
- Smolen J.S., Aletaha D., McInnes I.B. Rheumatoid arthritis. Lancet. 2016;388(10055):2023–2038. - PubMed
-
- Hazes J.M., Luime J.J. The epidemiology of early inflammatory arthritis. Nat Rev Rheumatol. 2011;7(7):381–390. - PubMed
-
- Adami G., Saag K.G. Osteoporosis pathophysiology, epidemiology, and screening in rheumatoid arthritis. Curr Rheumatol Rep. 2019;21(7):34. - PubMed
-
- Dimitroulas T., Nikas S.N., Trontzas P., Kitas G.D. Biologic therapies and systemic bone loss in rheumatoid arthritis. Autoimmun Rev. 2013;12(10):958–966. - PubMed
-
- Wang Q., Wang W., Peng X., Ge G., Li W., Zhu C., et al. Engineering peptide‐oligonucleotide nano‐conjugates via click reaction as self‐delivery system for gene therapy of inflammatory arthritis. Adv Funct Mater. 2024
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

