Bone marrow-derived mesenchymal stromal cells in necrotizing enterocolitis treatment: a narrative review
- PMID: 40822681
- PMCID: PMC12350335
- DOI: 10.3389/fped.2025.1624236
Bone marrow-derived mesenchymal stromal cells in necrotizing enterocolitis treatment: a narrative review
Abstract
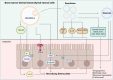
Necrotizing enterocolitis (NEC) presents a life-threatening intestinal emergency primarily affecting premature infants in neonatal intensive care units. This disease is a significant cause of morbidity and mortality in such newborns. NEC involves inflammation, bacterial overgrowth, and cell death affecting a portion of the bowel wall, commonly the distal ileum. Despite advances in neonatal care, the pathogenesis of NEC remains not fully understood. Although its pathogenesis remains not fully elucidated, the upregulation of Toll-like receptor 4 in the premature intestinal epithelium is recognized as a key factor contributing to epithelial barrier dysfunction. Recent studies have explored the potential of mesenchymal stromal cells (MSCs) in NEC management. MSCs are up-and-coming candidates for preclinical NEC models as they possess anti-inflammatory and immune modulatory properties, which reduce inflammation, help increase intestinal integrity, and help tissue repair. Bone marrow-derived mesenchymal stromal cells (BM-MSCs) have proven impactful in most experimental settings, mitigating injury from NEC and facilitating intestinal development. While MSC therapies hold promise, challenges remain regarding inconsistent isolation and expansion of these cells, variable differentiation, and possible tumorigenicity in vivo. As a result, the focus has been drawn to MSC-derived secretome, especially exosomes, as a novel cell-free therapeutic. These bioactive molecules transported by exosomes can reduce inflammation and facilitate tissue repair, providing a safer and more plausible alternative to treating NEC. Further research is needed to standardize secretome production and evaluate its clinical efficacy and safety. This review aims to provide a comprehensive overview of the mechanisms of action and the available research on human (h)BM-MSCs to support the development of studies that may prevent and/or treat the disease.
Keywords: bone marrow-derived mesenchymal stromal cells; exosomes; necrotizing enterocolitis; secretome; toll-like receptor 4.
© 2025 Tomaselli, Tripodi, Provitera, Raffaeli, Crippa, Raymo, Bronzoni, Santi, Arribas, Fumagalli, Loukogeorgakis, Ester Bernardo, Garrido and Cavallaro.
Conflict of interest statement
The authors declare that the research was conducted without any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials

