Dentate gyrus granule cell activation following extracellular electrical stimulation: a multi-scale computational model to guide hippocampal neurostimulation strategies
- PMID: 40822710
- PMCID: PMC12354454
- DOI: 10.3389/fncom.2025.1638002
Dentate gyrus granule cell activation following extracellular electrical stimulation: a multi-scale computational model to guide hippocampal neurostimulation strategies
Abstract
Introduction: The effectiveness of neural interfacing devices depends on the anatomical and physiological properties of the target region. Multielectrode arrays, used for neural recording and stimulation, are influenced by electrode placement and stimulation parameters, which critically impact tissue response. This study presents a multiscale computational model that predicts responses of neurons in the hippocampus-a key brain structure primarily involved in memory formation, especially the conversion of short-term memories into long-term storage-to extracellular electrical stimulation, providing insights into the effects of electrode positioning and stimulation strategies on neuronal response.
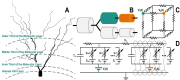
Methods: We modeled the rat hippocampus with highly detailed axonal projections, integrating the Admittance Method to model propagation of the electric field in the tissue with the NEURON simulation platform. The resulting model simulates electric fields generated by virtual electrodes in the perforant path of entorhinal cortical (EC) axons projecting to the dentate gyrus (DG) and predicts DG granule cell activation via synaptic inputs.
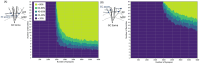
Results: We determined stimulation amplitude thresholds required for granule cell activation at different electrode placements along the perforant path. Membrane potential changes during synaptic activation were validated against experimental recordings. Additionally, we assessed the effects of bipolar electrode placements and stimulation amplitudes on direct and indirect activation.
Conclusion: Stimulation amplitudes above 750 μA consistently activate DG granule cells. Lower stimulation amplitudes are required for axonal activation and downstream synaptic transmission when electrodes are placed in the molecular layer, infra-pyramidal region, and DG crest.
Significance: The study and underlying methodology provide useful insights to guide the stimulation protocol required to activate DG granule cells following the stimulation of EC axons; the complete realistic 3D model presented constitutes an invaluable tool to strengthen our understanding of hippocampal response to electrical stimulation and guide the development and placement of prospective stimulation devices and strategies.
Keywords: admittance method; electrode design; extracellular electrical stimulation; multiscale modeling; neuron.
Copyright © 2025 Farzad, Wei, Bouteiller and Lazzi.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures






Similar articles
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
A Novel Design of a Portable Birdcage via Meander Line Antenna (MLA) to Lower Beta Amyloid (Aβ) in Alzheimer's Disease.IEEE J Transl Eng Health Med. 2025 Apr 10;13:158-173. doi: 10.1109/JTEHM.2025.3559693. eCollection 2025. IEEE J Transl Eng Health Med. 2025. PMID: 40657533 Free PMC article.
-
Deep brain and cortical stimulation for epilepsy.Cochrane Database Syst Rev. 2017 Jul 18;7(7):CD008497. doi: 10.1002/14651858.CD008497.pub3. Cochrane Database Syst Rev. 2017. PMID: 28718878 Free PMC article.
-
Non-invasive brain stimulation techniques for chronic pain.Cochrane Database Syst Rev. 2018 Mar 16;3(3):CD008208. doi: 10.1002/14651858.CD008208.pub4. Cochrane Database Syst Rev. 2018. Update in: Cochrane Database Syst Rev. 2018 Apr 13;4:CD008208. doi: 10.1002/14651858.CD008208.pub5. PMID: 29547226 Free PMC article. Updated.
References
-
- Ascoli G. A., Krichmar J. L. (2000). L-neuron: a modeling tool for the efficient generation and parsimonious description of dendritic morphology. Neurocomputing 32, 1003–1011. 10.1016/S0925-2312(00)00272-1 - DOI
LinkOut - more resources
Full Text Sources

