Delivered baicalein immunomodulatory hydrogel with dual properties of pH-responsive and anti-infection orchestrates pro-regenerative response of macrophages for enhanced hypertrophic scars therapy
- PMID: 40822925
- PMCID: PMC12355094
- DOI: 10.1016/j.mtbio.2025.102160
Delivered baicalein immunomodulatory hydrogel with dual properties of pH-responsive and anti-infection orchestrates pro-regenerative response of macrophages for enhanced hypertrophic scars therapy
Abstract
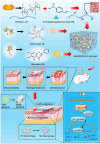
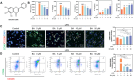
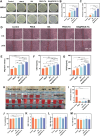
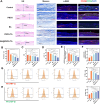
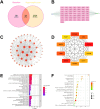
Antibiotic-resistant bacterial infections in skin wounds can cause persistent inflammatory responses, which may lead to severe hypertrophic scarring. In this study, a pH-responsive antibacterial hydrogel composed of phenylboronic acid-grafted chitosan (PBCS) and tannic acid (TA) was developed to achieve controlled and long-lasting release of baicalein (BA) to address the critical challenges of bacterial infection, wound healing, and scarring. The composite hydrogel (BA@PBCS-TA) not only demonstrates excellent injectability, self-healing properties, and robust mechanical performance but also exhibits favorable biological characteristics. Through pH-responsive release of BA, it effectively eliminates Methicillin-resistant Staphylococcus aureus (MRSA). In vivo experiments further confirm its ability to significantly inhibit fibroblast activation and reduce abnormal collagen deposition, effectively preventing excessive scar formation. Additionally, network pharmacology has identified Glycogen Synthase Kinase 3 Beta (GSK3β) as a key target for BA in inhibiting hypertrophic scar formation. Cellular experiments further demonstrate that the BA@PBCS-TA hydrogel can suppress GSK3β expression, activate the Wnt/β-catenin signaling pathway to repolarize macrophages into the M2 phenotype, and exhibit significant immunomodulatory effects. These results highlight the BA@PBCS-TA hydrogel's ability to harness the excellent properties of biomaterials and optimize BA's pharmacological effects, ultimately promoting wound healing and offering a strategic solution for scar reduction.
Keywords: Bacterial infection; Baicalein; GSK3β; Hydrogel; Hypertrophic scars; M2 macrophage.
© 2025 The Authors. Published by Elsevier Ltd.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
ROS/pH Dual-Responsive Hydrogel Dressings Loaded with Amphiphilic Structured Nano Micelles for the Repair of Infected Wounds.Int J Nanomedicine. 2025 Jun 23;20:8119-8142. doi: 10.2147/IJN.S522589. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40584783 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Laser therapy for treating hypertrophic and keloid scars.Cochrane Database Syst Rev. 2022 Sep 26;9(9):CD011642. doi: 10.1002/14651858.CD011642.pub2. Cochrane Database Syst Rev. 2022. PMID: 36161591 Free PMC article.
-
Silicone gel sheeting for treating hypertrophic scars.Cochrane Database Syst Rev. 2021 Sep 26;9(9):CD013357. doi: 10.1002/14651858.CD013357.pub2. Cochrane Database Syst Rev. 2021. PMID: 34564840 Free PMC article.
-
Regulating inflammation microenvironment and tenogenic differentiation as sequential therapy promotes tendon healing in diabetic rats.J Orthop Translat. 2025 Jun 5;53:63-81. doi: 10.1016/j.jot.2025.04.015. eCollection 2025 Jul. J Orthop Translat. 2025. PMID: 40529900 Free PMC article.
References
-
- Tripathi S., Soni K., Agrawal P., Gour V., Mondal R., Soni V. Hypertrophic scars and keloids: a review and current treatment modalities. Biomed. Dermatol. 2020;4(1):11. doi: 10.1186/s41702-020-00063-8. - DOI
-
- Zhang T., Wang X.F., Wang Z.C., Lou D., Fang Q.Q., Hu Y.Y., Zhao W.Y., Zhang L.Y., Wu L.H., Tan W.Q. Current potential therapeutic strategies targeting the TGF-β/Smad signaling pathway to attenuate keloid and hypertrophic scar formation. Biomed. Pharmacother. 2020;129 doi: 10.1016/j.biopha.2020.110287. - DOI - PubMed
LinkOut - more resources
Full Text Sources

