Influence of oropharyngeal therapy with mother's own milk on the microbiome and metabolome of very preterm infants: a pilot study
- PMID: 40823026
- PMCID: PMC12355658
- DOI: 10.3389/fnut.2025.1647379
Influence of oropharyngeal therapy with mother's own milk on the microbiome and metabolome of very preterm infants: a pilot study
Abstract
Background: Oropharyngeal therapy with mother's own milk (OPT-MOM) may serve as a promising therapeutic approach to elicit immunoprotective and anti-inflammatory benefits for preterm infants.
Objectives: This prospective pilot study aims to investigate whether OPT-MOM alters the oral microbiota, gut microbiota and metabolic profiles in very preterm infants.
Methods: The eligible infants were divided into two groups: the OPT-MOM group and the control group. The OPT-MOM group received oropharyngeal administration with mother's own milk every 3 h, starting within the first 48 h after birth and lasted for 14 days. Salivary samples and fecal samples from both groups were collected to detect microbes using 16S rRNA gene sequencing, while fecal metabolomics was measured by untargeted liquid chromatograph-mass spectrometer.
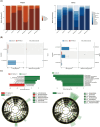
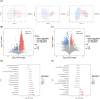
Results: A total of 26 very preterm infants were enrolled in the study, with 13 assigned to each group. Our study identified distinct oral and intestinal microbiome profiles in OPT-MOM group compared to the control group. Briefly, the relative abundance of the Escherichia-Shigella and Enterobacter genera was significantly reduced in the oral cavity of preterm infants in the OPT-MOM group, while the abundance of the Rothia genus increased markedly. After 14 days of intervention, the gut microbiota of preterm infants in the OPT-MOM group exhibited a significant decrease in the abundance of the Proteobacteria phylum and a concomitant increase in the abundance of the Firmicutes phylum, which emerged as the dominant phylum. Additionally, the OPT-MOM group showed a significant increase in the relative abundance of Streptococcus and Staphylococcus genus, while a significant decrease in Enterococcus and Enterobacter genus abundance was observed in the gut microbiota. The predominant bacteria in the oral microbiota of preterm infants are highly similar to those in the intestinal microbiota. Metabolomic profiling identified that the OPT-MOM group demonstrated significantly higher levels of multiple potentially beneficial metabolites, including N-acetylneuraminic acid, myristoylcarnitine, lauroylcarnitine, acetylcarnitine, and 2,4-dihydroxybutanoic acid.
Conclusion: Administration of OPT-MOM could promote the establishment of favorable microbial communities in both oral and intestinal ecosystems of preterm infants, potentially facilitating the production of metabolites that are crucial for infant health.
Keywords: breast milk; intestinal microbiota; metabolome; oral microbiota; preterm infants.
Copyright © 2025 Xiu, Yang, Lin, Zhang and Chen.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Similar articles
-
Early life bifidobacterial mother-infant transmission: greater contribution from the infant gut to human milk revealed by microbiomic and culture-based methods.mSystems. 2025 Jul 22;10(7):e0048025. doi: 10.1128/msystems.00480-25. Epub 2025 Jun 25. mSystems. 2025. PMID: 40558046 Free PMC article.
-
A Systemic Review of the Difference Between Diets for Preterm Infants Containing Raw Mother's Own Milk and Frozen or Pasteurized Mother's Own Milk.J Hum Lact. 2024 May;40(2):259-269. doi: 10.1177/08903344241227941. Epub 2024 Feb 8. J Hum Lact. 2024. PMID: 38328919
-
Changes in intestinal bacterial characteristics during hospitalization in the NICU in very low birth weight infants.Ital J Pediatr. 2025 Aug 26;51(1):263. doi: 10.1186/s13052-025-02095-4. Ital J Pediatr. 2025. PMID: 40859345 Free PMC article.
-
Alterations in vaginal microbiome in women with short cervix: longitudinal study of microbial diversity and impact of vaginal progesterone treatment.Ultrasound Obstet Gynecol. 2025 Aug;66(2):217-225. doi: 10.1002/uog.29269. Epub 2025 Jun 26. Ultrasound Obstet Gynecol. 2025. PMID: 40569025 Free PMC article.
-
Oropharyngeal colostrum in preventing mortality and morbidity in preterm infants.Cochrane Database Syst Rev. 2018 Sep 7;9(9):CD011921. doi: 10.1002/14651858.CD011921.pub2. Cochrane Database Syst Rev. 2018. PMID: 30191961 Free PMC article.
References
LinkOut - more resources
Full Text Sources

