Intervention of Astragalus Membranaceus Extract in rats of spinal cord injury: a systematic review and meta-analysis
- PMID: 40823287
- PMCID: PMC12353725
- DOI: 10.3389/fneur.2025.1637608
Intervention of Astragalus Membranaceus Extract in rats of spinal cord injury: a systematic review and meta-analysis
Abstract
Objective: Spinal cord injury (SCI) causes motor, sensory and autonomic dysfunction below the level of injury and its incidence is increasing every year. Astragalus Membranaceus Extract (AME) has received attention in spinal cord injury in recent years, but its specific effects in spinal cord injury are unclear.
Methods: Databases of PubMed, Embase, WOS, Cochrane Library, FMRS, Clinical trial, CNKI, VIP, and WangFang were searched from their establishment to December 1, 2024 using the following terms: "Astragalus propinquus," "Huang qi," "Astragalus mongholicus Bunge," "Spinal cord injuries," "spinal cord diseases," "spinal cord trauma." To ensure comprehensiveness, the search strategy included both traditional names (Astragalus) and scientific names (Astragalus membranaceus). Only studies published in Chinese or English were included. Cross-sectional studies, survey designs, quality improvement studies, and other study designs that did not meet the inclusion criteria were excluded.
Results: After screening, a total of 16 studies with 996 animals were included in the review. Astragalus Membranaceus Extract (AME) administration was associated with more significant functional recovery (mean difference [MD] = 3.68, 95% CI = 2.74, 4.62). Subgroup analyses showed the best functional recovery of the spinal cord when the dose exceeded 20 units and the duration of treatment was less than 14 days.
Conclusion: Our study suggests that AME has therapeutic potential for spinal cord injured rats. Further studies are needed to determine if this can be developed into a new alternative therapy through experimental and clinical studies with larger samples.
Systematic review registration: Identifier: CRD42024623721, website: https://www.crd.york.ac.uk/PROSPERO/.
Keywords: Huang qi; animal experiments; meta-analysis; phytomedicine; spinal cord injury.
Copyright © 2025 Wu, Yu, Yang, Li, Deng, Zhou, Tao, Chen, Zhang and Zhang.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


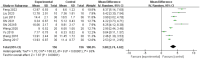



Similar articles
-
Oral Astragalus (Huang qi) for preventing frequent episodes of acute respiratory tract infection in children.Cochrane Database Syst Rev. 2016 Dec 1;12(12):CD011958. doi: 10.1002/14651858.CD011958.pub2. Cochrane Database Syst Rev. 2016. PMID: 27905672 Free PMC article.
-
Management of faecal incontinence and constipation in adults with central neurological diseases.Cochrane Database Syst Rev. 2013 Dec 18;(12):CD002115. doi: 10.1002/14651858.CD002115.pub4. Cochrane Database Syst Rev. 2013. Update in: Cochrane Database Syst Rev. 2014 Jan 13;(1):CD002115. doi: 10.1002/14651858.CD002115.pub5. PMID: 24347087 Updated.
-
Management of faecal incontinence and constipation in adults with central neurological diseases.Cochrane Database Syst Rev. 2014 Jan 13;2014(1):CD002115. doi: 10.1002/14651858.CD002115.pub5. Cochrane Database Syst Rev. 2014. Update in: Cochrane Database Syst Rev. 2024 Oct 29;10:CD002115. doi: 10.1002/14651858.CD002115.pub6. PMID: 24420006 Free PMC article. Updated.
-
Efficacy of Astragalus Membranaceus (Huang Qi) for Cancer-Related Fatigue: A Systematic Review and Meta-Analysis of Randomized Controlled Studies.Integr Cancer Ther. 2025 Jan-Dec;24:15347354241313344. doi: 10.1177/15347354241313344. Epub 2025 Apr 29. Integr Cancer Ther. 2025. PMID: 40302232 Free PMC article.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
References
Publication types
LinkOut - more resources
Full Text Sources

