Autologous stem cell transplantation in NK/T-cell lymphoma: Prognostic impact of EBV-DNA in a multinational cohort-A study by the EBMT Lymphoma Working Party
- PMID: 40823316
- PMCID: PMC12355192
- DOI: 10.1002/hem3.70184
Autologous stem cell transplantation in NK/T-cell lymphoma: Prognostic impact of EBV-DNA in a multinational cohort-A study by the EBMT Lymphoma Working Party
Abstract
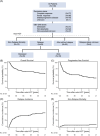
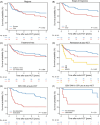
Natural killer (NK)/T-cell lymphomas (NKTCLs) are rare, aggressive lymphomas prevalent in East Asia and South America. Despite improvements, largely due to asparaginase-based therapies, outcomes for advanced disease remain poor, and the role of autologous stem cell transplantation (auto-HCT) remains controversial. This study evaluated real-world outcomes of auto-HCT in NKTCL patients across Asia and Europe. We included 130 adult NKTCL patients undergoing auto-HCT between 2011 and 2022 using data from the European Society for Blood and Marrow Transplantation (EBMT) registry and South Korean registry data. Median age was 51.3 years; 66.9% were male. Most patients (95.1%) had an Eastern Cooperative Oncology Group (ECOG) score 0-1; 65.3% had Stage III-IV disease. One prior therapy line was reported in 53.1%, and ≥2 lines in 46.9%. Asparaginase-based regimens were used in 79.5% pretransplant. Responses at auto-HCT included complete (59.7%), partial (27.9%) remission, and stable/progressive disease (12.4%). Epstein-Barr virus (EBV)-DNA in the peripheral blood was reported in 37.3%. With a median follow-up of 4.6 years, 3-year overall survival (OS) and progression-free survival (PFS) were 63.8% and 47.6%. Relapse and NRM rates at 3 years were 46.7% and 5.7%. Patients in complete remission had improved 3-year OS (75.2%) compared to PR (52.8%) and stable/progressive disease (32.0%) (P = 0.007). Detectable EBV-DNA in the blood at auto-HCT was associated with poor outcomes (3-year OS: 26.7% vs. 78.1% in patients with undetectable EBV-DNA; P < 0.0001). Patients achieving complete remission and undetectable EBV-DNA in the blood before auto-HCT had a favorable survival, suggesting auto-HCT may be a treatment option in selected high-risk patients. This is the largest multinational cohort evaluating prognostic factors for auto-HCT for NKTCL.
© 2025 The Author(s). HemaSphere published by John Wiley & Sons Ltd on behalf of European Hematology Association.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
