A Combined Experimental and Modeling Workflow to Tune Surface Properties of Organic Materials via Cocrystallization
- PMID: 40823385
- PMCID: PMC12355690
- DOI: 10.1021/acs.chemmater.5c00634
A Combined Experimental and Modeling Workflow to Tune Surface Properties of Organic Materials via Cocrystallization
Abstract
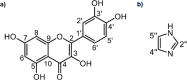
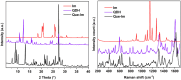
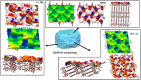
Cocrystallization is a specific crystal engineering strategy widely used to enhance the dissolution rate or bioavailability of active pharmaceutical ingredients. In this work, we demonstrate how cocrystallization can also be used to tune surface properties of crystalline particles, such as facet-specific surface chemistry, polarity, and wettability. As a model system, we have isolated a cocrystal of quercetin (Que) with imidazole (Im). Que is widely recognized for its potential antioxidative and antibacterial properties and other potentially beneficial therapeutic effects. Surface chemistry is a property that can affect ease of manufacturability (e.g., flowability) and storage stability (e.g., tendency to agglomerate) for particulate materials; here, we used cocrystallization to modify this property for Que particles. The screening of suitable coformers was first performed in silico using a method based on molecular complementarity and hydrogen bond (H-bond) propensity scores. Experiments were conducted using the identified coformers via slurrying in different solvents. The cocrystal was identified and characterized by powder X-ray diffraction (PXRD), differential scanning calorimetry (DSC), thermogravimetric analysis (TGA), Raman spectroscopy, and solid-state nuclear magnetic resonance (SSNMR). The Que-Im crystal structure was solved by single-crystal X-ray diffraction (SXRD) and characterized computationally, using the attachment energy model, and experimentally by contact angle measurements. Structural and vibrational analyses showed a major modification in intermolecular interactions of Que-Im compared to pure Que polymorphs. The contribution of the H-bond and π-π stacking interactions to the crystal energy is similar, but the crystal morphology exposes a predominant facet growing via van der Waals interactions. As a result, Que-Im is more hydrophobic than the dihydrate (QDH) and dimethyl sulfoxide (QDMSO) solvate forms. The shift in the average water droplet contact angle from 38.8 ± 1.0° (QDMSO), 48.0 ± 3.2° (QDH) to 78.5 ± 3.9° (Que-Im) is strong evidence of a marked decrease in hydrophilicity of the target compound.
© 2025 The Authors. Published by American Chemical Society.
Figures










References
-
- Montis R., Hursthouse M. B., Kendrick J., Howe J., Whitby R. J.. Combining Structural Rugosity and Crystal Packing Comparison: A Route to More Polymorphs? Cryst. Growth Des. 2022;22(1):559–569. doi: 10.1021/acs.cgd.1c01132. - DOI
-
- Simone E., Cenzato M. V., Nagy Z. K.. A Study on the Effect of the Polymeric Additive HPMC on Morphology and Polymorphism of Ortho-Aminobenzoic Acid Crystals. J. Cryst. Growth. 2016;446:50–59. doi: 10.1016/j.jcrysgro.2016.04.034. - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
