Quality of antidiabetic medicines in 13 sub-Saharan African countries: a cross-sectional survey
- PMID: 40823495
- PMCID: PMC12355408
- DOI: 10.1016/j.eclinm.2025.103405
Quality of antidiabetic medicines in 13 sub-Saharan African countries: a cross-sectional survey
Abstract
Background: The burden of diabetes is rising dramatically in low- and middle-income countries. The menace of substandard and falsified drugs constitutes a major hazard that compromises healthcare. The DIABDAF study aimed to assess the quality of routinely used antidiabetic drugs including oral drugs and insulins in sub-Saharan Africa.
Methods: Drugs were collected in 13 sub-Saharan African cities in licensed and unlicensed places of sales between February 2020 and March 2023. Chemical analyses were conducted blindly in a public laboratory following recommended good laboratory practices. Drug quality was classified based on the ratio of measured to expected active ingredient dosage: 95-105% as good (A), 85-94·99% or 105·01-115% as low (B), and below 85% or above 115% as very low (C). Impurity levels were assessed using thresholds from the United States and European Pharmacopoeias monographs.
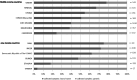
Findings: A convenient samples of 4951 antidiabetic drugs were collected from 13 sub-Saharan African countries (Seven middle-income and six low-income countries). Out of the 1673 (of 4951 collected) drug samples randomly tested, 28·0% (n: 468, 95% CI [22·3-33·0]) failed to meet standards related to the expected content of active ingredients (B: 27·2% 95% CI [21·5-32·0]; C: 0·8% 95% CI [0·2-3·5]), with more samples showing underdosage (19·31% 95% CI [14·8-24·3]) than overdosage (8·67% 95% CI [5·3-12·5]). Impurity levels were excessive in 9·68% (n: 162, 95% CI [6·0-14·8]) of samples. Overall, 32·8% (n: 548, 95% CI [26·5-38·1]) were deemed to be of poor quality according to active ingredient content or impurity level. In multivariate logistic regression, factors associated with worse quality were drugs, expired status, and country of purchase.
Interpretation: In this multinational study assessing the quality of antidiabetic drugs in sub-Saharan Africa, we found a significant proportion of poor-quality drugs. National health authorities must take action to ensure access to safe, high-quality medications for diabetic patients.
Funding: DIABDAF study was exclusively supported by French public grant (INSERM, AVIESAN, AP-HP, and University of Paris Cité).
Keywords: Africa; Antidiabetic agents; Counterfeit drugs; Diabetes; Drug quality; Drugs; Falsified drugs; Insulin; Low- and middle-income countries; Substandard.
© 2025 The Authors.
Conflict of interest statement
We declare no competing interests.
Figures


References
-
- International Diabetes Federation . International Diabetes Federation; 2025. Diabetes Atlas of the International Diabetes Federation [Internet]diabetesatlas.org Available from:
-
- Atun R., Davies J.I., Gale E.A.M., et al. Diabetes in Sub-Saharan Africa: from clinical care to health policy. Lancet Diabetes Endocrinol. 2017;5(8):622–667. - PubMed
-
- World Health Organization . World Health Organization; Geneva: 2016. Global Report on Diabetes [Internet] p. 83.https://iris.who.int/handle/10665/204871 Available from:
-
- Ward Z.J., Yeh J.M., Reddy C.L., et al. Estimating the total incidence of type 1 diabetes in children and adolescents aged 0-19 years from 1990 to 2050: a global simulation-based analysis. Lancet Diabetes Endocrinol. 2022;10(12):848–858. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

