Management of Clostridioides difficile infection in patients with haematological malignancies and after cellular therapy: guidelines from 10th European Conference on Infections in Leukaemia (ECIL-10)
- PMID: 40823499
- PMCID: PMC12355421
- DOI: 10.1016/j.eclinm.2025.103371
Management of Clostridioides difficile infection in patients with haematological malignancies and after cellular therapy: guidelines from 10th European Conference on Infections in Leukaemia (ECIL-10)
Abstract
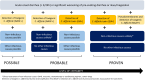
Clostridioides difficile infection (CDI) poses a significant challenge in patients with haematological malignancies (HM) and those undergoing cellular therapy such as haematopoietic cell transplantation (HCT) or CAR T-cell therapy. These patients have high rates of both colonization with Clostridioides difficile and diarrhoea due to non-infectious causes, leading to challenges with establishing diagnosis and optimal management of CDI, especially in the setting of molecular detection of toxin genes alone. Current severity criteria are of limited usefulness since underlying haematological disease and its treatment impact white blood count and inflammatory manifestations of severe CDI. Extensive exposure to antibiotics, profound microbiota damage and bidirectional relationship with gastro-intestinal graft-versus-host disease after transplant further complicate clinical management. Therefore, the 10th European Conference on Infections in Leukemia (ECIL-10) group comprehensively reviewed the literature (published 01/01/2010-15/09/2024) on the epidemiology, treatment and prevention of CDI, and formulated consensus recommendations for the management of CDI specific to this population. New definitions of proven, probable and possible CDI in this population were developed and proposed for use in clinical research to standardise reporting.
Keywords: Clostridioides difficile; Faecal microbiota transplantation; Fidaxomicin; Haematological malignancies; Hematopoietic stem cell transplantation; Vancomycin.
© 2025 The Authors.
Conflict of interest statement
ER received honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Tillots and Cepheid and support for attending meetings and/or travel from Tillots and Mundipharma. LG received honoraria for lectures, presentations, speakers bureaus, manuscript writing or educational events from Takeda, Gilead, Pfizer, Abbvie, Novartis, Servier, BMS, support for attending meetings and/or travel from Gilead, Roche, Servier, Swixx, Abbvie, Pfizer, and Participation on a Data Safety Monitoring Board or Advisory Board from Roche, Gilead, Pfizer, Takeda, Abbvie, BMS. BWT reports a grant to institution for an investigator-initiated trial from Seqirus and payment to institution for advisory board participation from Moderna. None as support for the present manuscript. All other authors: nothing to declare.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources