Efficacy and Safety of Oral Spironolactone for Women With Acne Vulgaris: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials With Trial Sequential Analysis
- PMID: 40823723
- PMCID: PMC12359290
- DOI: 10.1111/jocd.70411
Efficacy and Safety of Oral Spironolactone for Women With Acne Vulgaris: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials With Trial Sequential Analysis
Abstract
Background: Adult acne vulgaris is a chronic inflammatory skin condition that primarily affects females. Initial management includes topical and oral medications, but important limitations include ineffectiveness, nonadherence, and adverse effects. Spironolactone has shown good results in off-label acne management.
Aims: We aim to conduct a systematic review and meta-analysis exploring the safety and efficacy of oral spironolactone for females with acne.
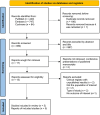
Methods: We searched PubMed, Embase, and Cochrane for randomized controlled trials (RCTs) comparing oral spironolactone in women with acne to placebo. The primary endpoint was the objective assessment of acne improvement. Secondary endpoints included subjective assessment and adverse events. Statistical analysis was performed using Review Manager 5.4. Heterogeneity was assessed with I2 statistics.
Results: We included 563 patients from 5 RCTs, of which 251 (42.9%) received spironolactone. Objective assessment of acne improvement (OR 6.59; 95% 3.50-12.43; p < 0.00001; I2 = 0%) was sixfold higher in the spironolactone group compared with placebo. Subjective assessment showed no difference between the two groups (OR 5.22; 95% 0.62-44.24; p < 0.13; I2 = 85%). Menstrual irregularities (OR 1.09; 95% 0.37-3.25; p = 0.88; I2 = 33%) and breast enlargement (OR 1.37; 95% 0.79-2.38; p = 0.26; I2 = 0%) were nonsignificant in patients taking spironolactone. Trial sequential analysis (TSA) confirmed that the required sample size was reached, favoring spironolactone over placebo.
Conclusion: Our study suggests that oral spironolactone improves acne in female patients compared to placebo without increasing risks; thus, it should be elevated from "off-label" use to an officially recommended standard of care.
Prospero registration: CRD42024626984.
Keywords: acne vulgaris; spironolactone; women.
© 2025 The Author(s). Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


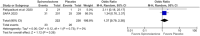
Similar articles
-
Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis.Cochrane Database Syst Rev. 2017 Dec 22;12(12):CD011535. doi: 10.1002/14651858.CD011535.pub2. Cochrane Database Syst Rev. 2017. Update in: Cochrane Database Syst Rev. 2020 Jan 9;1:CD011535. doi: 10.1002/14651858.CD011535.pub3. PMID: 29271481 Free PMC article. Updated.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Interventions for acne scars.Cochrane Database Syst Rev. 2016 Apr 3;4(4):CD011946. doi: 10.1002/14651858.CD011946.pub2. Cochrane Database Syst Rev. 2016. PMID: 27038134 Free PMC article.
-
Complementary therapies for acne vulgaris.Cochrane Database Syst Rev. 2015 Jan 19;1(1):CD009436. doi: 10.1002/14651858.CD009436.pub2. Cochrane Database Syst Rev. 2015. PMID: 25597924 Free PMC article.
-
Minocycline for acne vulgaris: efficacy and safety.Cochrane Database Syst Rev. 2003;(1):CD002086. doi: 10.1002/14651858.CD002086. Cochrane Database Syst Rev. 2003. Update in: Cochrane Database Syst Rev. 2012 Aug 15;(8):CD002086. doi: 10.1002/14651858.CD002086.pub2. PMID: 12535427 Updated.
References
-
- National Institute for Health and Care Excellence (NICE) , “Acne Vulgaris: Management,” (2021), https://www.nice.org.uk/guidance/ng198/chapter/Recommendations. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

