Efficacy and Safety of Oral Spironolactone for Women With Acne Vulgaris: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials With Trial Sequential Analysis
- PMID: 40823723
- PMCID: PMC12359290
- DOI: 10.1111/jocd.70411
Efficacy and Safety of Oral Spironolactone for Women With Acne Vulgaris: A Systematic Review and Meta-Analysis of Randomized Placebo-Controlled Trials With Trial Sequential Analysis
Abstract
Background: Adult acne vulgaris is a chronic inflammatory skin condition that primarily affects females. Initial management includes topical and oral medications, but important limitations include ineffectiveness, nonadherence, and adverse effects. Spironolactone has shown good results in off-label acne management.
Aims: We aim to conduct a systematic review and meta-analysis exploring the safety and efficacy of oral spironolactone for females with acne.
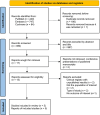
Methods: We searched PubMed, Embase, and Cochrane for randomized controlled trials (RCTs) comparing oral spironolactone in women with acne to placebo. The primary endpoint was the objective assessment of acne improvement. Secondary endpoints included subjective assessment and adverse events. Statistical analysis was performed using Review Manager 5.4. Heterogeneity was assessed with I2 statistics.
Results: We included 563 patients from 5 RCTs, of which 251 (42.9%) received spironolactone. Objective assessment of acne improvement (OR 6.59; 95% 3.50-12.43; p < 0.00001; I2 = 0%) was sixfold higher in the spironolactone group compared with placebo. Subjective assessment showed no difference between the two groups (OR 5.22; 95% 0.62-44.24; p < 0.13; I2 = 85%). Menstrual irregularities (OR 1.09; 95% 0.37-3.25; p = 0.88; I2 = 33%) and breast enlargement (OR 1.37; 95% 0.79-2.38; p = 0.26; I2 = 0%) were nonsignificant in patients taking spironolactone. Trial sequential analysis (TSA) confirmed that the required sample size was reached, favoring spironolactone over placebo.
Conclusion: Our study suggests that oral spironolactone improves acne in female patients compared to placebo without increasing risks; thus, it should be elevated from "off-label" use to an officially recommended standard of care.
Prospero registration: CRD42024626984.
Keywords: acne vulgaris; spironolactone; women.
© 2025 The Author(s). Journal of Cosmetic Dermatology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


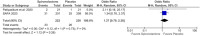
References
-
- National Institute for Health and Care Excellence (NICE) , “Acne Vulgaris: Management,” (2021), https://www.nice.org.uk/guidance/ng198/chapter/Recommendations. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

