Alterations of NMDAR Subunits in the Cerebrospinal Fluid Across Neurodegenerative and Immunological Disorders
- PMID: 40823742
- PMCID: PMC12359294
- DOI: 10.1111/jnc.70192
Alterations of NMDAR Subunits in the Cerebrospinal Fluid Across Neurodegenerative and Immunological Disorders
Abstract
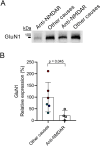
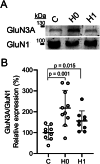
N-methyl-D-aspartate receptors (NMDARs) are glutamate-binding calcium channels that play a key role in brain function and have been linked to many neurological disorders. NMDARs are multi-pass membrane heterotetrameric complexes composed of two compulsory GluN1 subunits and two GluN2 (A-D) or GluN3 (A-B) subunits, from which GluN1, GluN2B, GluN2A, and GluN3A are widely expressed in the adult brain. This study assesses the presence of GluN1, GluN2B, GluN2A, and GluN3A in the cerebrospinal fluid (CSF) from healthy individuals, viral and autoimmune encephalitis, Huntington's disease (HD) and Alzheimer's disease (AD) patients. Samples were run in SDS-PAGE under reducing conditions and resolved with different anti-ectodomain and anti-C-terminal antibodies that combined with immunoprecipitation analyses, served to demonstrate the presence of full-length GluN1, GluN2A, GluN2B, and GluN3A in CSF. These NMDAR subunit complexes are not associated with extracellular vesicles. As a proof of concept of the identity of NMDAR subunits in the CSF, we demonstrated reduced levels of GluN1 in the CSF from patients with autoimmune encephalitis caused by anti-GluN1 antibodies compared with other causes of encephalitis; and showed a depletion of CSF GluN3A in a Grin3a knockout mouse model. Moreover, we observed higher GluN3A levels in CSF in both asymptomatic and symptomatic HD patients; while GluN2A levels were lower in CSF from AD patients. In conclusion, here we demonstrate the presence of NMDAR full-length subunits in CSF and that changes in NMDAR subunits balance could serve to identify alterations related to pathological conditions.
Keywords: Alzheimer's disease; CSF; GluN1; GluN2A; GluN2B; GluN3A; Huntington's disease; NMDAR; biomarker.
© 2025 The Author(s). Journal of Neurochemistry published by John Wiley & Sons Ltd on behalf of International Society for Neurochemistry.
Conflict of interest statement
The authors have nothing to report.
The authors have stated explicitly that there are no conflicts of interest in connection with this article. APa received grant support from the Ministry of Health (MINSAL) and the Ministry of Education, Research and University (MIUR), from CARIPLO Foundation; personal compensation as a consultant/scientific advisory board member for Biogen, Lundbeck, Roche, Nutricia, General Healthcare (GE). APi received consultancy/speaker fees from Abbvie, Angelini, Bial, Lundbeck, Roche, and Zambon pharmaceuticals. H.Z. has served on scientific advisory boards and/or as a consultant for Abbvie, Acumen, Alector, Alzinova, ALZpath, Amylyx, Annexon, Apellis, Artery Therapeutics, AZTherapies, Cognito Therapeutics, CogRx, Denali, Eisai, LabCorp, Merry Life, Nervgen, Novo Nordisk, Optoceutics, Passage Bio, Pinteon Therapeutics, Prothena, Quanterix, Red Abbey Labs, reMYND, Roche, Samumed, Siemens Healthineers, Triplet Therapeutics, and Wave, has given lectures sponsored by Alzecure, BioArctic, Biogen, Cellectricon, Fujirebio, Lilly, Novo Nordisk, Roche, and WebMD, and is a co‐founder of Brain Biomarker Solutions in Gothenburg AB (BBS), which is a part of the GU Ventures Incubator Program (outside submitted work).
Figures



References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

