Enantioselective NHC-Catalyzed Annulation via Umpolung for the Construction of Hydroxylamine Architectures with Variable Ring Sizes
- PMID: 40823767
- PMCID: PMC12400417
- DOI: 10.1021/acs.orglett.5c02576
Enantioselective NHC-Catalyzed Annulation via Umpolung for the Construction of Hydroxylamine Architectures with Variable Ring Sizes
Abstract
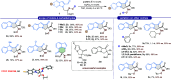
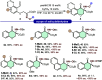
An efficient and highly enantioselective organocatalytic annulation for the synthesis of chiral hydroxylamine-containing heterocycles is described. The N-heterocyclic carbene (NHC)-catalyzed reaction successfully engages traditionally inert keto-oxime ethers as electrophiles in an intramolecular cyclization with in situ generated Breslow intermediates. This protocol overcomes the inherent challenges of this substrate class, such as low C═N bond electrophilicity and potential N-O bond cleavage, to deliver diverse five- and six-membered N-O frameworks in excellent yields (up to 99%) and enantioselectivities (up to >99% ee). This work provides a powerful, metal-free strategy to access valuable chiral building blocks and significantly expands the reactivity scope of keto-oximes in asymmetric catalysis.
Figures

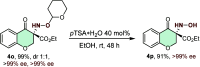
Similar articles
-
Induced-Fit Chiral N-Heterocyclic Carbene Ligands for Asymmetric Catalysis.Acc Chem Res. 2025 Jul 1;58(13):2157-2177. doi: 10.1021/acs.accounts.5c00304. Epub 2025 Jun 19. Acc Chem Res. 2025. PMID: 40536020
-
The Role of Anions in Guanidinium-Catalyzed Chiral Cation Ion Pair Catalysis.Acc Chem Res. 2025 Jul 15;58(14):2269-2281. doi: 10.1021/acs.accounts.5c00283. Epub 2025 Jun 30. Acc Chem Res. 2025. PMID: 40587427
-
From Mono-N-Protected Amino Acids to Pyridones: A Decade of Evolution of Bifunctional Ligands for Pd(II)-Catalyzed C-H Activation.Acc Chem Res. 2025 Aug 29. doi: 10.1021/acs.accounts.5c00503. Online ahead of print. Acc Chem Res. 2025. PMID: 40878662
-
Asymmetric Synthesis of Bioactive Molecules for Pesticide Development with N-Heterocyclic Carbene (NHC)-Organocatalysis.Chem Asian J. 2025 Aug 9:e00720. doi: 10.1002/asia.202500720. Online ahead of print. Chem Asian J. 2025. PMID: 40783791 Review.
-
Primary Amine-Based Photoclick Chemistry: From Concept to Diverse Applications in Chemical Biology and Medicinal Chemistry.Acc Chem Res. 2025 Jul 1;58(13):1963-1981. doi: 10.1021/acs.accounts.5c00158. Epub 2025 Jun 18. Acc Chem Res. 2025. PMID: 40532071 Review.
References
-
- Bertrand S., Helesbeux J.-J., Larcher G., Duval O.. Hydroxamate, a Key Pharmacophore Exhibiting a Wide Range of Biological Activities. Mini-Rev. Med. Chem. 2013;13:1311–1326. doi: 10.2174/13895575113139990007. - DOI - PubMed
- Hydroxamic Acids; Gupta, S. P. , Ed.; Springer-Verlag: 2013.
- The Chemistry of Hydroxylamines, Oximes and Hydroxamic Acids; Rappoport, Z. , Liebman, J. F. , Eds.; John Wiley & Sons, Ltd.: 2008.
- Papp-Wallace K. M., Nguyen N. Q., Jacobs M. R., Bethel C. R., Barnes M. D., Kumar V., Bajaksouzian S., Rudin S. D., Rather P. N., Bhavsar S., Ravikumar T., Deshpande P. K., Patil V., Yeole R., Bhagwat S. S., Patel M. V., van der Akker F., Bonomo R. A.. Strategic approaches to overcome resistance against gram-negative pathogens using β-lactamase inhibitors and β-lactam enhancers: activity of three novel diazabicyclooctanes WCK 5153, Zidebactam (WCK 5107), and WCK 4234. J. Med. Chem. 2018;61:4067–4086. doi: 10.1021/acs.jmedchem.8b00091. - DOI - PMC - PubMed
- Lambert K. M., Cox J. B., Liu L., Jackson A. C., Yruegas S., Wiberg K. B., Wood J. L.. Total synthesis of (±)-Phyllantidine: development and mechanistic evaluation of a ring expansion for installation of embedded nitrogen-Oxygen Bonds. Angew. Chem., Int. Ed. 2020;59:9757–9766. doi: 10.1002/anie.202003829. - DOI - PMC - PubMed
-
- Blakemore D. C., Castro L., Churcher I., Rees D. C., Thomas A. W., Wilson D. M., Wood A.. Organic synthesis provides opportunities to transform drug discovery. Nat. Chem. 2018;10:383–394. doi: 10.1038/s41557-018-0021-z. - DOI - PubMed
- Cabre A., Verdaguer X., Riera A.. Recent advances in the enantioselective synthesis of chiral amines via transition metal-catalyzed asymmetric hydrogenation. Chem. Rev. 2022;122:269–339. doi: 10.1021/acs.chemrev.1c00496. - DOI - PMC - PubMed
-
- Jenul C., Sieber S., Daeppen C., Mathew A., Lardi M., Pessi G., Hoepfner D., Neuburger M., Linden A., Gademann K., Eberl L.. Biosynthesis of fragin is controlled by a novel quorum sensing signal. Nat. Commun. 2018;9:1297. doi: 10.1038/s41467-018-03690-2. - DOI - PMC - PubMed
- Sieber S., Mathew A., Jenul C., Kohler T., Bar M., Carrion V. J., Cazorla F. M., Stalder U., Hsieh Y.-C., Bigler L., Eberl L., Gadermann K.. Mitigation of Pseudomonas Syringae virulence by signal inactivation. Sci. Adv. 2021;7:eabg2293. doi: 10.1126/sciadv.abg2293. - DOI - PMC - PubMed
- Bode J. W.. Chemical protein synthesis with the α-ketoacid-hydroxylamine ligation. Acc. Chem. Res. 2017;50:2104–2115. doi: 10.1021/acs.accounts.7b00277. - DOI - PubMed
-
- Bach R. D., Schlegel H. B.. The Bond Dissociation Energy of the N-O Bond. J. Phys. Chem. A. 2021;125:5014–5021. doi: 10.1021/acs.jpca.1c02741. - DOI - PubMed
- Riddell F. G., Turner E. S.. The Barrier to Rotation about the N-O Bond. J. Chem. Soc. Perkin Trans. 2. 1978;2:707–708. doi: 10.1039/P29780000707. - DOI
- Raban M., Kost D.. Stereolabile Configurational Units and Inversional Stereochemistry in Sulfenamides and Hydroxylamines. Tetrahedron. 1984;40:3345–3381. doi: 10.1016/S0040-4020(01)91484-X. - DOI
- Dhanju S., Blazejewski B. W., Crich D.. Synthesis of Trialkylhydroxylamnes by Stepwise Reduction of O-acyl N,N-Disubstituted Hydroxylamines: Substituent Effects on the Reduction of O-(1-Acyloxyalkyl)hydroxylamines and on the Conformational Dynamics of N-Alkoxypiperidines. J. Org. Chem. 2017;82:5345–5353. doi: 10.1021/acs.joc.7b00717. - DOI - PubMed
-
- Mas-Rosello J., Cramer N.. Catalytic Reduction of Oximes to Hydroxylamines: Current Methods, Challenges and Opportunities. Chem. Eur. J. 2022;28:e202103683. doi: 10.1002/chem.202103683. - DOI - PMC - PubMed
- Lu B., Yu J., Zhang X., Chen G.-Q.. Recent Advances on Catalytic Asymmetric Hydrogenation of Oximes and Oxime Ethers. Tetrahedron Lett. 2024;136:154914. doi: 10.1016/j.tetlet.2024.154914. - DOI
LinkOut - more resources
Full Text Sources

