Peripheral heterochromatin tethering is required for chromatin-based nuclear mechanical response
- PMID: 40823810
- PMCID: PMC12359041
- DOI: 10.1093/nar/gkaf763
Peripheral heterochromatin tethering is required for chromatin-based nuclear mechanical response
Abstract
The cell nucleus is a mechanically responsive structure that governs how external forces affect chromosomes. Chromatin, particularly transcriptionally inactive heterochromatin, resists nuclear deformations through its mechanical response. However, chromatin also exhibits liquid-like properties, casting ambiguity on the physical mechanisms of chromatin-based nuclear elasticity. To determine how heterochromatin strengthens nuclear mechanical response, we performed polymer physics simulations of a nucleus model validated by micromechanical measurements and chromosome conformation capture data. The attachment of peripheral heterochromatin to the lamina is required to transmit forces directly to the chromatin and elicit its elastic response. Thus, increases in heterochromatin levels increase nuclear rigidity by increasing the linkages between chromatin and the lamina. Crosslinks within heterochromatin, such as HP1α proteins, can also stiffen nuclei, but only if chromatin is peripherally tethered. In contrast, heterochromatin affinity interactions that may drive liquid-liquid phase separation do not contribute to nuclear rigidity. When the nucleus is stretched, gel-like peripheral heterochromatin can bear stresses and deform, while the more fluid-like interior euchromatin is less perturbed. Thus, heterochromatin's internal structure and stiffness may regulate nuclear mechanics via peripheral attachment to the lamina, while also enabling nuclear mechanosensing of external forces and external measurement of the nucleus' internal architecture.
© The Author(s) 2025. Published by Oxford University Press.
Conflict of interest statement
None declared.
Figures
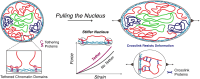



Update of
-
Peripheral heterochromatin tethering is required for chromatin-based nuclear mechanical response.bioRxiv [Preprint]. 2025 Feb 16:2025.02.12.637704. doi: 10.1101/2025.02.12.637704. bioRxiv. 2025. Update in: Nucleic Acids Res. 2025 Aug 11;53(15):gkaf763. doi: 10.1093/nar/gkaf763. PMID: 39990304 Free PMC article. Updated. Preprint.
Similar articles
-
Peripheral heterochromatin tethering is required for chromatin-based nuclear mechanical response.bioRxiv [Preprint]. 2025 Feb 16:2025.02.12.637704. doi: 10.1101/2025.02.12.637704. bioRxiv. 2025. Update in: Nucleic Acids Res. 2025 Aug 11;53(15):gkaf763. doi: 10.1093/nar/gkaf763. PMID: 39990304 Free PMC article. Updated. Preprint.
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Chromosome organization by one-sided and two-sided loop extrusion.Elife. 2020 Apr 6;9:e53558. doi: 10.7554/eLife.53558. Elife. 2020. PMID: 32250245 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Healthcare workers' informal uses of mobile phones and other mobile devices to support their work: a qualitative evidence synthesis.Cochrane Database Syst Rev. 2024 Aug 27;8(8):CD015705. doi: 10.1002/14651858.CD015705.pub2. Cochrane Database Syst Rev. 2024. PMID: 39189465 Free PMC article.
Cited by
-
Differential Crosslinking and Contractile Motors Drive Nuclear Chromatin Compaction.bioRxiv [Preprint]. 2025 Jul 27:2025.07.24.666416. doi: 10.1101/2025.07.24.666416. bioRxiv. 2025. PMID: 40777249 Free PMC article. Preprint.
-
Differential Crosslinking and Contractile Motors Drive Nuclear Chromatin Compaction.ArXiv [Preprint]. 2025 Jul 23:arXiv:2507.17883v1. ArXiv. 2025. PMID: 40740516 Free PMC article. Preprint.
References
Grants and funding
LinkOut - more resources
Full Text Sources

