A brain-derived tau oligomer polymorph is associated with cognitive resilience to Alzheimer's disease
- PMID: 40823851
- PMCID: PMC12359067
- DOI: 10.1002/alz.70550
A brain-derived tau oligomer polymorph is associated with cognitive resilience to Alzheimer's disease
Abstract
Introduction: Misfolded tau can assemble into oligomers that adopt distinct conformations, referred to as polymorphs, each with unique biochemical and pathological properties. These tau polymorphs are thought to influence disease progression in Alzheimer's disease (AD) and related disorders. Interestingly, some individuals with AD pathology remain cognitively intact (non-demented with Alzheimer's neuropathology [NDAN]), suggesting potential differences in tau polymorph profiles.
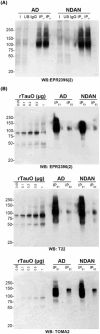
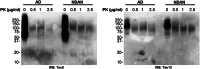
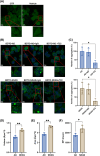
Methods: Brain-derived tau oligomers (BDTOs) were isolated from post mortem hippocampi of AD and NDAN individuals. Their biophysical, biochemical, and functional properties were assessed via protease digestion, immunocharacterization, atomic force microscopy, tau seeding in biosensor cells, hippocampal slice electrophysiology, and SH-SY5Y toxicity assays.
Results: NDAN-BDTOs exhibited protease resistance, different conformational profiles, formed larger aggregates, preserved synaptic function, and reduced neuronal toxicity compared to AD-BDTOs.
Discussion: The data suggests that a structurally stable yet less toxic tau polymorph in NDAN may underlie cognitive resilience, supporting the therapeutic relevance of targeting specific tau polymorphs.
Highlights: Non-demented with Alzheimer's neuropathology (NDAN) brain-derived tau oligomers (BDTOs) exhibit distinct, more stable structural polymorphs compared to those from Alzheimer's disease (AD). NDAN-BDTOs are larger, more protease resistant, and less toxic in cell and hippocampal slice models. Both NDAN- and AD-BDTOs seed tau aggregation, but NDAN-BDTOs promote formation of less toxic assemblies. Structural stability of NDAN-BDTOs may contribute to cognitive resilience despite AD pathology. Mimicking non-toxic tau polymorphs could represent a novel therapeutic strategy for AD.
Keywords: Alzheimer's disease; brain‐derived tau oligomers; cognitive resilience; non‐demented with Alzheimer's neuropathology; tau polymorphs.
© 2025 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

