Gut microbiota-mediated betaine regulates skeletal muscle fiber type transition by affecting m6A RNA methylation and Myh7 expression
- PMID: 40824213
- PMCID: PMC12363516
- DOI: 10.1080/19490976.2025.2545434
Gut microbiota-mediated betaine regulates skeletal muscle fiber type transition by affecting m6A RNA methylation and Myh7 expression
Abstract
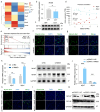
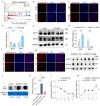
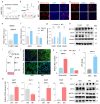
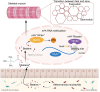
Skeletal muscle fiber composition is essential for maintaining muscle function and overall health. Growing evidence underscores the pivotal role of the gut-muscle axis in mediating the influence of gut microbiota on skeletal muscle development. However, the mechanisms underlying microbiota-mediated regulation of skeletal muscle fiber type remain unclear. Here, we employed multi-omics approaches, including RNA-seq, MeRIP-seq, 16S rRNA gene sequencing, and metabolomics, to investigate the causal relationship between the gut microbiota and skeletal muscle fiber transition. Our results demonstrate that the gut microbiota modulates skeletal muscle fiber transition by influencing N6-methyladenosine (m6A) methylation to regulate the expression of the slow-twitch fiber marker Myh7. Specifically, METTL3-dependent m6A methylation enhances Myh7 gene expression, leading to an increased proportion of slow-twitch fibers and a reduction in fast-twitch fibers. Furthermore, the microbiota-derived methyl donor betaine promotes Myh7 expression and Akkermansia muciniphila (AKK) abundance, and facilitates fast-to-slow fiber conversion via m6A modification. The transplantation of AKK significantly altered betaine levels and m6A modification, thereby promoting muscle fiber remodeling. In conclusion, these findings reveal that AKK-coordinated betaine drives skeletal muscle fiber conversion by modulating Myh7 mRNA expression. This study provides novel insights into the role of m6A RNA methylation in the gut-muscle crosstalk, highlighting potential therapeutic targets for muscle-related disorders.
Keywords: Betaine; Myh7; gut microbiota; m6A RNA methylation; myofiber-type transition.
Conflict of interest statement
No potential conflict of interest was reported by the author(s).
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
