Multifunctional 3D-Printed Wound Dressings Containing a Combination of Synergistic Antimicrobials in the Management of MRSA Infected Topical Wounds
- PMID: 40824249
- PMCID: PMC12400267
- DOI: 10.1021/acsami.5c08968
Multifunctional 3D-Printed Wound Dressings Containing a Combination of Synergistic Antimicrobials in the Management of MRSA Infected Topical Wounds
Abstract
Despite increased pre- and postoperative care and aseptic practices in surgical rooms, methicillin-resistant Staphylococcus aureus (MRSA) continues to colonize acute surgical wounds. MRSA is also present in chronic nonhealing wounds, such as diabetic foot and pressure ulcers. In this work, advanced antimicrobial-loaded wound dressings are 3D printed using fused deposition modeling. To achieve a high antimicrobial effect, the topical antiseptic octenidine (OCT) was incorporated into the pellets used in the feeder of the extruder prior to fused modeling. Lysostaphin (LYS), a lytic enzyme that cleaves MRSA peptidoglycan, was incorporated by supramolecular interactions on the surface of the OCT-loaded dressings to exploit the anti-MRSA synergy identified here between OCT and LYS showing a fractional inhibition concentration index (FICI) of 0.156. Minimum inhibitory concentration (MIC) and bactericidal concentration (MBC) values for the OCT were 1 and 25 μg/mL, respectively, whereas the MIC and MBC values for the LYS were 0.1 and 0.2 μg/mL, respectively. The resulting dressings completely eradicate MRSA USA 300 inocula (105 CFU/mL) in 96 h. The bactericidal mechanisms exerted by these dressings were identified through molecular techniques, showing lytic effects on the cell wall peptidoglycans of treated bacteria. Additionally, OCT at 1 μg/mL was able to reduce lipopolysaccharide (100 ng/mL)-induced NO production on murine J774A.1 macrophages by more than 90% demonstrating its simultaneous anti-inflammatory action. This effect was also corroborated by the qRT-PCR analysis of several pro-inflammatory genes including IL-1β, IL-6, TNF-α, and Nos2. The combination of OCT and LYS within the dressings reveals higher in vivo therapeutic effects compared to free compounds or individual antimicrobial-loaded dressings. In vitro and in preclinical models, the use of OCT-LYS dressings effectively reduces MRSA bioburden and inflammation, promoting fast wound healing.
Keywords: 3D printing; MRSA; antimicrobial therapies; wound dressings; wound healing.
Figures



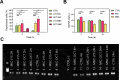
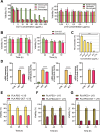


References
-
- Murray C. J. L., Ikuta K. S., Sharara F., Swetschinski L., Robles Aguilar G., Gray A., Han C., Bisignano C., Rao P., Wool E., Johnson S. C., Browne A. J., Chipeta M. G., Fell F., Hackett S., Haines-Woodhouse G., Kashef Hamadani B. H., Kumaran E. A. P., McManigal B., Achalapong S., Agarwal R., Akech S., Albertson S., Amuasi J., Andrews J., Aravkin A., Ashley E., Babin F.-X., Bailey F., Baker S., Basnyat B., Bekker A., Bender R., Berkley J. A., Bethou A., Bielicki J., Boonkasidecha S., Bukosia J., Carvalheiro C., Castañeda-Orjuela C., Chansamouth V., Chaurasia S., Chiurchiù S., Chowdhury F., Clotaire Donatien R., Cook A. J., Cooper B., Cressey T. R., Criollo-Mora E., Cunningham M., Darboe S., Day N. P. J., De Luca M., Dokova K., Dramowski A., Dunachie S. J., Duong Bich T., Eckmanns T., Eibach D., Emami A., Feasey N., Fisher-Pearson N., Forrest K., Garcia C., Garrett D., Gastmeier P., Giref A. Z., Greer R. C., Gupta V., Haller S., Haselbeck A., Hay S. I., Holm M., Hopkins S., Hsia Y., Iregbu K. C., Jacobs J., Jarovsky D., Javanmardi F., Jenney A. W. J., Khorana M., Khusuwan S., Kissoon N., Kobeissi E., Kostyanev T., Krapp F., Krumkamp R., Kumar A., Kyu H. H., Lim C., Lim K., Limmathurotsakul D., Loftus M. J., Lunn M., Ma J., Manoharan A., Marks F., May J., Mayxay M., Mturi N., Munera-Huertas T., Musicha P., Musila L. A., Mussi-Pinhata M. M., Naidu R. N., Nakamura T., Nanavati R., Nangia S., Newton P., Ngoun C., Novotney A., Nwakanma D., Obiero C. W., Ochoa T. J., Olivas-Martinez A., Olliaro P., Ooko E., Ortiz-Brizuela E., Ounchanum P., Pak G. D., Paredes J. L., Peleg A. Y., Perrone C., Phe T., Phommasone K., Plakkal N., Ponce-de-Leon A., Raad M., Ramdin T., Rattanavong S., Riddell A., Roberts T., Robotham J. V., Roca A., Rosenthal V. D., Rudd K. E., Russell N., Sader H. S., Saengchan W., Schnall J., Scott J. A. G., Seekaew S., Sharland M., Shivamallappa M., Sifuentes-Osornio J., Simpson A. J., Steenkeste N., Stewardson A. J., Stoeva T., Tasak N., Thaiprakong A., Thwaites G., Tigoi C., Turner C., Turner P., van Doorn H. R., Velaphi S., Vongpradith A., Vongsouvath M., Vu H., Walsh T., Walson J. L., Waner S., Wangrangsimakul T., Wannapinij P., Wozniak T., Young Sharma T. E. M. W., Yu K. C., Zheng P., Sartorius B., Lopez A. D., Stergachis A., Moore C., Dolecek C., Naghavi M.. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet. 2022;399(10325):629–655. doi: 10.1016/S0140-6736(21)02724-0. - DOI - PMC - PubMed
-
- Scharn C. R., Tickler I. A., Tenover F. C., Goering R. V.. Characterization of SCC Mec Instability in Methicillin-Resistant Staphylococcus Aureus Affecting Adjacent Chromosomal Regions, Including the Gene for Staphylococcal Protein A (Spa) Antimicrob. Agents Chemother. 2022;66(4):e0237421. doi: 10.1128/aac.02374-21. - DOI - PMC - PubMed
-
- Merk, H. ; Diaz Högberg, L. ; Plachouras, D. ; Suetens, C. ; Monnet, D. L. . Assessing the Health Burden of Infections with Antibiotic-Resistant Bacteria in the EU/EEA, 2016–2020. ECDC: Stockholm, 2020; 10.2900/73460. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

