Biannual Mass Azithromycin Distributions for Preschool Children and Malaria Parasitemia: A Secondary Analysis of the MORDOR Cluster Randomized Trial
- PMID: 40824641
- PMCID: PMC12362227
- DOI: 10.1001/jamanetworkopen.2025.27148
Biannual Mass Azithromycin Distributions for Preschool Children and Malaria Parasitemia: A Secondary Analysis of the MORDOR Cluster Randomized Trial
Abstract
Importance: Mass azithromycin distributions may reduce malaria parasitemia in the short term, but longer-term effectiveness is unclear.
Objective: To examine whether biannual mass azithromycin distributions are associated with lower rates of malaria parasitemia in preschool children living in Niger.
Design, setting, and participants: A cluster randomized trial was performed from November 23, 2014, until June 9, 2020, as an ancillary trial to a larger trial studying the effect of mass azithromycin on child mortality. Study communities (ie, government-defined health catchment areas) in Niger were randomized in a 1:1 ratio to biannual (ie, twice-yearly) mass administration of azithromycin or placebo to all children aged 1 to 59 months and followed up for 5 years. Data analyses were performed from June 25, 2023, to April 27, 2025.
Intervention: Twice-yearly administration of a single dose of oral azithromycin, 20 mg/kg, or placebo.
Main outcomes and measures: The prevalence of parasitemia 4 years after the community started treatment, assessed in a random sample of 40 children per community.
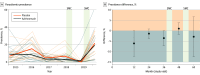
Results: Among the 30 communities in Niger included in the study at baseline, the 15 communities randomized to azithromycin consisted of 1695 children (mean [SD] age, 30.8 [2.8] months; 858 [51.8%] male) and the 15 communities randomized to placebo consisted of 3031 children (mean [SD] age, 30.6 [2.6] months; 157 [52.0%] male). The mean prevalence of malaria parasitemia at baseline was 8.9% (95% CI, 5.1%-15.7%) in the azithromycin arm and 6.7% (95% CI, 4.0%-12.6%) in the placebo arm. At annual follow-up visits up until month 48, parasitemia was not statistically significantly lower in the azithromycin arm compared with the placebo arm, assuming a 10% prevalence in the placebo arm (-3.3 percentage points [PP]; 95% CI, -5.8 to -0.2 PP; permutation P = .05). The Niger Ministry of Health instituted seasonal malaria chemoprevention (SMC) after the month 36 study visit. Analysis restricted to the period before SMC found significantly less parasitemia in the azithromycin arm compared with the placebo arm (4.8 PP lower; 95% CI, -7.4 to -1.3 PP; permutation P = .02).
Conclusions and relevance: In this placebo-controlled cluster randomized trial, malaria among children aged 1 to 59 months was lower in communities treated with biannual mass azithromycin, but the effect was significant only for the first 3 years of the trial, before SMC.
Trial registration: ClinicalTrials.gov Identifier: NCT02048007.
Conflict of interest statement
Figures

References
-
- World Health Organization . WHO Guideline on Mass Drug Administration of Azithromycin to Children Under Five Years of Age to Promote Child Survival. Published 2020. Accessed June 30, 2025. https://www.who.int/publications/i/item/9789240009585 - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

