Cytomegalovirus prophylaxis with letermovir in pediatric (birth to <18 years of age) hematopoietic cell transplant recipients: pharmacokinetics, efficacy, and safety results of a Phase 2b study
- PMID: 40824644
- PMCID: PMC12486802
- DOI: 10.1128/aac.00420-25
Cytomegalovirus prophylaxis with letermovir in pediatric (birth to <18 years of age) hematopoietic cell transplant recipients: pharmacokinetics, efficacy, and safety results of a Phase 2b study
Abstract
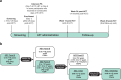
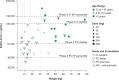
Letermovir, a cytomegalovirus (CMV) terminase complex inhibitor, was first approved for prophylaxis of CMV infection and disease in adult CMV-seropositive allogeneic hematopoietic cell transplant (HCT) recipients (R+). This study evaluated the pharmacokinetics (PK), efficacy, and safety of letermovir in pediatric R+ allogeneic HCT recipients. In this Phase 2b, single-arm, open-label study, 65 participants were enrolled sequentially in three age groups (AG; AG1, 12 to <18 years; AG2, 2 to <12 years; and AG3, birth to <2 years). PK was evaluated in an initial cohort in each AG using intensive PK data to confirm or modify dosing before enrolling the remaining participants. Adult HCT population PK (PopPK) data were used to establish the exposure reference range. The adult letermovir dose evaluated in AG1 and AG2 participants achieved exposures generally within the adult HCT reference range. In AG3, the initial cohort (letermovir with cyclosporin A) achieved exposures trending lower than the median exposure target; the letermovir dose was increased for the remaining participants. Efficacy and safety in pediatric participants were generally consistent with adult HCT data. A pediatric HCT PopPK model was developed to determine dose recommendations to be included in patient prescribing information. The doses evaluated achieved exposures generally within the adult HCT reference range. At exposures achieved, letermovir was efficacious and safe in preventing clinically significant CMV infection in pediatric allogeneic HCT recipients. The observed concentration data informed a pediatric PopPK model to optimize final letermovir dose recommendations in this population.CLINICAL TRIALSThis study is registered with ClinicalTrials.gov as NCT03940586.
Keywords: cytomegalovirus; hematopoietic cell transplantation; letermovir; pediatric; pharmacokinetics.
Conflict of interest statement
D.B. has participated in advisory boards for Alexion and served on speakers' bureau of Jazz Pharmaceuticals. L.D.-I. has received research support from AiCuris, Ansun BioPharma, Astellas, MSD, and Pfizer, and is a consultant for Astellas, MSD, and Takeda. C.J.F. has participated in advisory boards for Amgen, Jazz Pharmaceuticals, and Link Healthcare. A.H.G. has participated in advisory boards for Novartis and Sanofi. A.H.G. has received research support from Gilead Sciences, MSD, and Pfizer; is a consultant for Amplyx, Astellas, Basilea, F2G, Gilead Sciences, MSD, and Pfizer; and served on the speakers' bureau of Astellas, Basilea, F2G, Gilead Sciences, MSD, and Pfizer. N.B., B.H., C.L.G., M.P., J.B.M., and C.B. are current employees of Merck Sharp & Dohme LLC, a subsidiary of Merck & Co., Inc., Rahway, NJ, USA, and may own stock and/or stock options in Merck & Co., Inc., Rahway, NJ, USA. The other authors declare no conflict of interest.
Figures

References
-
- Passweg JR, Baldomero H, Ciceri F, Corbacioglu S, de la Cámara R, Dolstra H, Glass B, Greco R, McLornan DP, Neven B, de Latour RP, Perić Z, Ruggeri A, Snowden JA, Sureda A. 2023. Hematopoietic cell transplantation and cellular therapies in Europe 2021. The second year of the SARS-CoV-2 pandemic. a report from the EBMT activity survey. Bone Marrow Transplant 58:647–658. doi: 10.1038/s41409-023-01943-3 - DOI - PMC - PubMed
-
- Center for International Blood and Marrow Transplant Research . 2022. HRSA transplant activity report: number of HCTs performed in the United States and reported to CIBMTR, by year and age group, 2016–2020
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

