Footprints of Worldwide Adaptation in Structured Populations of Drosophila melanogaster Through the Expanded DEST 2.0 Genomic Resource
- PMID: 40824865
- PMCID: PMC12360290
- DOI: 10.1093/molbev/msaf132
Footprints of Worldwide Adaptation in Structured Populations of Drosophila melanogaster Through the Expanded DEST 2.0 Genomic Resource
Abstract
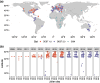
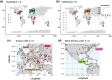
Large-scale genomic resources can place genetic variation into an ecologically informed context. To advance our understanding of the population genetics of the fruit fly Drosophila melanogaster, we present an expanded release of the community-generated population genomics resource Drosophila Evolution over Space and Time (DEST 2.0; https://dest.bio/). This release includes 530 high-quality pooled libraries from flies collected across six continents over more than a decade (2009 to 2021), most at multiple time points per year; 211 of these libraries are sequenced and shared here for the first time. We used this enhanced resource to elucidate several aspects of the species' demographic history and identify novel signs of adaptation across spatial and temporal dimensions. For example, we showed that the spatial genetic structure of populations is stable over time, but that drift due to seasonal contractions of population size causes populations to diverge over time. We identified signals of adaptation that vary between continents in genomic regions associated with xenobiotic resistance, consistent with independent adaptation to common pesticides. Moreover, by analyzing samples collected during spring and fall across Europe, we provide new evidence for seasonal adaptation related to loci associated with pathogen response. Furthermore, we have also released an updated version of the DEST genome browser. This is a useful tool for studying spatiotemporal patterns of genetic variation in this classic model system.
Keywords: Drosophila melanogaster; dataset; ecological genomics; local adaptation; population structure; seasonal selection.
© The Author(s) 2025. Published by Oxford University Press on behalf of Society for Molecular Biology and Evolution.
Figures







References
MeSH terms
Grants and funding
- University of Vermont
- 101059238/Horizon Europe project FAIRiCUBE
- 101059238/Horizon Europe project FAIRiCUBE
- 2R35GM11816506/Horizon Europe project FAIRiCUBE
- 31003A-182262/SNSF_/Swiss National Science Foundation/Switzerland
- 310030_219283/SNSF_/Swiss National Science Foundation/Switzerland
- FZEB-0-214654/SNSF_/Swiss National Science Foundation/Switzerland
- R35 GM119686/NH/NIH HHS/United States
- 2145688/NH/NIH HHS/United States
- PID2020-115874GB-I00/NH/NIH HHS/United States
- MICIU/AEI /10.13039/501100011033/NH/NIH HHS/United States
- PID2023-148838NB-I00/NH/NIH HHS/United States
- MICIU/AEI/10.13039/501100011033/NH/NIH HHS/United States
- 2021 SGR 00417/NH/NIH HHS/United States
- Departament de Recerca i Universitats
- MCIN/AEI/10.13039/501100011033/Ministerio de Ciencia e Innovación of Spain
- PID2020-113168GB-I00/Ministerio de Ciencia e Innovación of Spain
- 2021SGR00279/Comissió Interdepartamental de Recerca I Innovació Tecnològica
- 451-03-66/2024-03/200007/Ministry of Science
- PID2021-127107NB-I00/Ministry of Science
- 10.55776/P32935/Austrian Science Funds
- 10.55776/P33734/Austrian Science Funds
- FR2973/11-1/German Science Foundation
- BB/T007516/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- QINV0196-IINV529010100/Pontificia Universidad Católica del Ecuador
- VR-2015-04680/Pontificia Universidad Católica del Ecuador
- VR-2020-05412/Pontificia Universidad Católica del Ecuador
- 255619725/Deutsche Forschungsgemeinschaft
- 503272152/Deutsche Forschungsgemeinschaft
- 322980/Academy of Finland project
- 451-03-65/2024-03/ 200178/Academy of Finland project
- 451-03-47/2023-01/200178/Academy of Finland project
- NE/V001566/1/NERC
- #61-1673/Bettencourt Schueller Foundation
- CRI Core Research Fellowship
- PID2020-119255GB-I00/Jane Coffin Childs Memorial Fund for Medical Research
- SEV-2015-0533/Ministry of Economy and Competitiveness
- CEX2019-000917/Ministry of Economy and Competitiveness
- European Regional Development Fund
- DP190102512/Australian Research Council
- ALT 248-02018/EMBO
- ANR-20-CE02-011-01/ANR
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

