Identifying microbial functional guilds performing cryptic organotrophic and lithotrophic redox cycles in anaerobic granular biofilms
- PMID: 40824903
- PMCID: PMC12360541
- DOI: 10.1371/journal.pone.0330380
Identifying microbial functional guilds performing cryptic organotrophic and lithotrophic redox cycles in anaerobic granular biofilms
Abstract
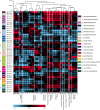
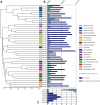
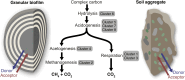
Granular biofilms used in anaerobic digester systems contain diverse microbial populations that interact to hydrolyze organic matter and produce methane within controlled environments. Prior research investigated the feasibility of utilizing granular biofilms obtained from an anaerobic digester to remove nitrate without the addition of exogenous electron donors. These granules possessed a unique structure of alternating light and dark iron sulfide and pyrite rich layers that potentially served as both an electron source and sink, linking carbon, nitrogen, sulfur, and iron cycles. To characterize the functional roles of diverse microbial populations enriched within these layered biofilms, we analyzed metagenomes obtained from three different granules. Comparisons between the functional gene content of forty metagenome assembled genomes (MAGs) identified phylogenetically cohesive functional guilds. Each of these functional MAG clusters was assigned to specific steps in anaerobic digestion (hydrolysis, acidogenesis, acetogenesis, and methanogenesis) and anaerobic respiration (denitrification and sulfate reduction). Comparisons with metagenomes derived from a variety of natural and engineered ecosystems confirmed that the enriched denitrifying bacteria were similar to populations typically found in wetlands and biological nitrogen removal systems. Analysis of read alignments to individual genes within the forty MAGs identified conserved genomic features that were representative of the functions that distinguished functional guilds. Overall, this research illustrates the utility of functional based classification of microorganisms for characterizing ecosystem functions and highlights the potential application of engineered ecosystems to serve as experimental models for complex natural ecosystems.
Copyright: © 2025 Flinkstrom et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Environment selected microbial function rather than taxonomic species in a plateau saline-alkaline wetland.Appl Environ Microbiol. 2025 Jul 23;91(7):e0220624. doi: 10.1128/aem.02206-24. Epub 2025 Jul 3. Appl Environ Microbiol. 2025. PMID: 40607849 Free PMC article.
-
Short-Term Memory Impairment.2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2024 Jun 8. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 31424720 Free Books & Documents.
-
Rehabilitation following surgery for lumbar spinal stenosis.Cochrane Database Syst Rev. 2013 Dec 9;2013(12):CD009644. doi: 10.1002/14651858.CD009644.pub2. Cochrane Database Syst Rev. 2013. PMID: 24323844 Free PMC article.
-
Carbon dioxide detection for diagnosis of inadvertent respiratory tract placement of enterogastric tubes in children.Cochrane Database Syst Rev. 2025 Feb 19;2(2):CD011196. doi: 10.1002/14651858.CD011196.pub2. Cochrane Database Syst Rev. 2025. PMID: 39968844
References
-
- McHugh S, O’Reilly C, Mahony T, Colleran E, O’Flaherty V. Anaerobic Granular Sludge Bioreactor Technology. Rev Environ Sci Technol. 2003;2:225–45.
-
- Skiadas IV, Gavala HN, Schmidt JE, Ahring BK. Anaerobic Granular Sludge and Biofilm Reactors. In: Ahring BK, Ahring BK, Angelidaki I, Dolfing J, EUegaard L, Gavala HN, editors. Biomethanation II. Berlin, Heidelberg: Springer Berlin Heidelberg. 2003. p. 35–67. - PubMed
-
- O’Flaherty V, Collins G, Mahony T. The Microbiology and Biochemistry of Anaerobic Bioreactors with Relevance to Domestic Sewage Treatment. Rev Environ Sci Biotechnol. 2006;5(1):39–55. doi: 10.1007/s11157-005-5478-8 - DOI
LinkOut - more resources
Full Text Sources
Research Materials

