Curcumin inhibits ferroptosis through dessuccinylation of SIRT5-associated ACSL4 protein, and plays a chondroprotective role in osteoarthritis
- PMID: 40825027
- PMCID: PMC12360603
- DOI: 10.1371/journal.pone.0328139
Curcumin inhibits ferroptosis through dessuccinylation of SIRT5-associated ACSL4 protein, and plays a chondroprotective role in osteoarthritis
Abstract
Background: Ferroptosis of chondrocytes plays a crucial role in the progression of osteoarthritis (OA). This study aimed to explore the role of curcumin (Cur) in interfering with chondrocyte ferroptosis in OA.
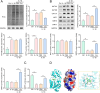
Methods: Rat chondrocytes were treated with 10 ng/mL interleukin-1β (IL-1β) for 48 hours to mimic the OA microenvironment. The protective effects of Cur were evaluated in vitro by assessing cell viability and ferroptosis. Molecular docking was performed to validate the structural interaction between Cur and the SIRT5 protein. Co-immunoprecipitation (CO-IP) confirmed the binding relationship between SIRT5 and ACSL4. Additionally, the efficacy of Cur in alleviating OA progression was assessed in an in vivo OA rat model.
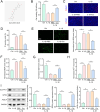
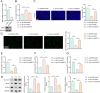
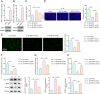
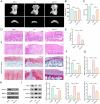
Results: Cur treatment significantly attenuated IL-1β-induced chondrocyte injury by enhancing cell viability and inhibiting ferroptosis. Cur also markedly reduced global protein lysine succinylation levels. IL-1β suppressed SIRT5 expression, while Cur treatment upregulated SIRT5 expression. The molecular structure of Cur exhibits strong complementarity with the SIRT5 protein, forming a stable complex with high binding affinity. Inhibition of SIRT5 attenuated the protective effects of Cur on chondrocytes and increased ACSL4 succinylation levels. SIRT5 physically interacted with ACSL4, and SIRT5-mediated desuccinylation of ACSL4 repressed its function, thereby mitigating ferroptosis. Cur alleviates OA progression in vivo by inhibiting cartilage destruction, bone erosion, and chondrocyte injury, and by smoothing subchondral bone surfaces.
Conclusion: Cur protects chondrocytes in vitro by inhibiting ferroptosis and suppresses cartilage degeneration and bone erosion in vivo, demonstrating a chondroprotective role in OA. These effects are mediated through SIRT5-dependent desuccinylation of ACSL4, which regulates ferroptosis pathways.
Copyright: © 2025 Xu et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Dehydrotanshinone II A alleviates osteoarthritis via activating PPARγ to inhibit ferroptosis in chondrocytes.Sci Rep. 2025 Aug 12;15(1):29602. doi: 10.1038/s41598-025-14896-y. Sci Rep. 2025. PMID: 40797087 Free PMC article.
-
Growth factor independence 1 ameliorates osteoarthritis by inhibiting chondrocyte ferroptosis via inactivation of MAPK signaling pathway.J Orthop Translat. 2025 Jul 29;54:101-114. doi: 10.1016/j.jot.2025.07.003. eCollection 2025 Sep. J Orthop Translat. 2025. PMID: 40787173 Free PMC article.
-
Duhuo jisheng decoction alleviates osteoarthritis progression by mitigating ferroptosis in chondrocytes via the nuclear factor erythroid 2-related factor 2/glutathione peroxidase 4 axis.J Ethnopharmacol. 2025 Aug 17;353(Pt B):120441. doi: 10.1016/j.jep.2025.120441. Online ahead of print. J Ethnopharmacol. 2025. PMID: 40829693
-
SIRT3 mitigates osteoarthritis by suppressing ferroptosis through activating AMPK signaling pathway.Cell Signal. 2025 Nov;135:112063. doi: 10.1016/j.cellsig.2025.112063. Epub 2025 Aug 14. Cell Signal. 2025. PMID: 40818538
-
WITHDRAWN: Non-aspirin, non-steroidal anti-inflammatory drugs for treating osteoarthritis of the knee.Cochrane Database Syst Rev. 2007 Jul 18;2006(1):CD000142. doi: 10.1002/14651858.CD000142.pub2. Cochrane Database Syst Rev. 2007. PMID: 17636601 Free PMC article.
References
LinkOut - more resources
Full Text Sources

