High-fat diet impairs intermediate-term memory by autophagic-lysosomal dysfunction in Drosophila
- PMID: 40825051
- PMCID: PMC12370196
- DOI: 10.1371/journal.pgen.1011818
High-fat diet impairs intermediate-term memory by autophagic-lysosomal dysfunction in Drosophila
Abstract
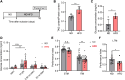
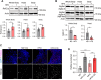
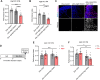
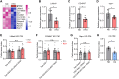
High-fat diet (HFD) is considered a risk factor for age-related memory impairments such as Alzheimer's disease. However, how HFD affects memory formation remains unclear. In this study, we established a model of memory defects caused by HFD in Drosophila. Our results revealed that the HFD impaired intermediate-term memory (ITM), but not short-term memory (STM), produced by classical aversive olfactory conditioning, and decreased autophagic activity in the heads of the HFD-fed flies. Transient reduction in autophagic activity also impaired ITM, but not STM. Genetic enhancement of autophagic activity in neurons effectively restored ITM performance in the HFD-fed flies. Mechanistically, HFD impairs lysosomal function by downregulating the expression of lysosome-related genes, leading to impaired fusion of autophagosomes with lysosomes. These findings suggest that HFD impairs ITM by reducing autophagic activity and lysosomal dysfunction in the neurons.
Copyright: © 2025 Yue et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

