A20 (TNFAIP3) Distinguishes Attack From Remission in Pediatric Patients With Monophasic MOGAD
- PMID: 40825157
- PMCID: PMC12363411
- DOI: 10.1212/NXI.0000000000200452
A20 (TNFAIP3) Distinguishes Attack From Remission in Pediatric Patients With Monophasic MOGAD
Abstract
Background and objectives: Acquired demyelinating syndromes associated with serum antibodies against myelin oligodendrocyte glycoprotein have been recognized as MOG-IgG-associated disorders (MOGADs). Patients with MOGAD show distinct features compared with individuals with multiple sclerosis (MS) or neuromyelitis optica spectrum disorders (NMOSDs). Up to 50% of patients experience relapsing disease courses, usually associated with persisting high MOG-IgG titers. However, further biomarkers are needed to discriminate monophasic from multiphasic MOGAD. Recently, lowered levels of tumor necrosis factor α-induced protein 3 (TNFAIP3, or A20) have been shown to be associated with attack in a small group of pediatric patients with MOGAD. The aim of this study was to evaluate A20 as a possible biomarker discriminating attack from remission in a larger cohort of pediatric patients with MOGAD.
Methods: In this cohort study, we tested 162 serum samples from 62 pediatric patients with MOGAD for A20 levels using commercially available ELISA kits. To compare A20 levels with those in non-MOGAD patients, we further included 46 serum samples from 37 pediatric patients with MS, NMOSD with AQP4-IgG, clinically isolated syndrome, or other neurologic disorders.
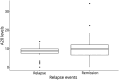
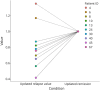
Results: In grouped analysis, A20 serum levels were significantly lower during attack compared with remission in patients with monophasic MOGAD. In grouped analysis of patients with multiphasic MOGAD, there was no such significant difference in A20 levels at attack vs remission. Among patients (n = 10) with paired attack and remission time points, there was a significant difference in A20 levels (p = 0.029). A20 levels were tendentially higher in patients on immunomodulatory treatments compared with untreated patients.
Discussion: Reflecting the anti-inflammatory role of A20, its relative decrease during attacks might even start before the patient's first symptoms. Thus, longitudinal evaluation of A20 at (yet to identifiable) standardized time points might have prognostic implications. Serum A20 levels in pediatric patients with MOGAD may help to distinguish attacks from remission in monophasic disease courses. Consequently, A20 needs to be prospectively investigated in standardized multicentric longitudinal study designs, with a focus on diagnostic, prognostic, and therapeutic implications.
Conflict of interest statement
C. Lechner, S. Saxena, and H. Lokhande report no disclosures relevant to the manuscript. M. Breu received speaker honoraria from Sanofi Genzyme. A. Eisenkölbl, M. Karenfort, A. Klein, S. Leiz, M. Preisel, T. Rooney, M. Rosso, M. Schimmel, and E. Wendel report no disclosures relevant to the manuscript. M. Reindl is supported by research grants from the Euroimmun, and Roche, and consulting fees and advisory board from Roche (to institution). Markus Reindl works at the Clinical Department of the Medical University of Innsbruck (Innsbruck, Austria), which offers diagnostic testing for MOG-IgG and other autoantibodies. M. Baumann received compensation for advisory boards and speaker honoraria from Novartis, Biogen, and Roche. K. Rostásy serves as consultant for Roche in Operetta II trial and received speaker honoraria from Merck. T. Chitnis has received consulting fees from Genentech-Roche and Novartis and research grants from Genentech-Roche, Bristol Myers Squibb, and Novartis. Go to
Figures
References
-
- Krupp LB, Tardieu M, Amato MP, et al. International Pediatric Multiple Sclerosis Study Group criteria for pediatric multiple sclerosis and immune-mediated central nervous system demyelinating disorders: revisions to the 2007 definitions. Mult Scler. 2013;19(10):1261-1267. doi: 10.1177/1352458513484547 - DOI - PubMed
-
- Bruijstens AL, Lechner C, Flet-Berliac L, et al. E.U. paediatric MOG consortium consensus: Part 1 - classification of clinical phenotypes of paediatric myelin oligodendrocyte glycoprotein antibody-associated disorders. Eur J Paediatr Neurol. 2020;29:2-13. doi: 10.1016/j.ejpn.2020.10.006 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
