Indole Propionic Acid Regulates Gut Immunity: Mechanisms of Metabolite-Driven Immunomodulation and Barrier Integrity
- PMID: 40825672
- PMCID: PMC12375546
- DOI: 10.4014/jmb.2503.03045
Indole Propionic Acid Regulates Gut Immunity: Mechanisms of Metabolite-Driven Immunomodulation and Barrier Integrity
Abstract
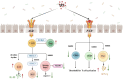
Indole propionic acid (IPA) is a functional indole derivative produced exclusively by intestinal flora through tryptophan metabolism. Numerous studies have shown that IPA has a variety of beneficial biological functions, including anti-inflammatory and antioxidant effects, immunomodulation, intestinal barrier protection, regulation of intestinal flora composition, and neuroprotection. IPA, as an intestinal microbial metabolite, actively participates in the establishment of intestinal immune homeostasis and positively influences the prevention and control of intestinal diseases, thereby playing an indispensable role in regulating host health. We conducted a comprehensive literature review to explore the synthesis of IPA in vivo, the mechanism of action on intestinal immunity, and the promise of its application in the treatment of related diseases. The physiological and biological effects of IPA were investigated to explore its potential application in future drug discovery. Obviously, IPA plays an important role in intestinal immunity and is effective in the treatment of related diseases. IPA helps regulate intestinal immune cell function, inhibiting inflammatory response and enhancing intestinal barrier function through its effects on the aryl hydrocarbon receptor, the pregnane X receptor, and other related signaling pathways. The development of IPA as a target drug for the treatment of intestinal diseases is promising. Although IPA research is still in the experimental animal model stage, there is growing interest in the many therapeutic applications of IPA and increasing opportunities to further modify IPA for future clinical applications.
Keywords: AhR/PXR; Indole propanoic acid; gut barrier integrity; intestinal immunity; microbiome-derived metabolites.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

