8-Methoxybicolosin C from Lespedeza bicolor Attenuates Inflammation and Oxidative Stress via Nrf2/HO-1 and NF-κB/MAPK Pathways in Lipopolysaccharide-Induced Mouse Kupffer Cells
- PMID: 40825682
- PMCID: PMC12375545
- DOI: 10.4014/jmb.2503.03013
8-Methoxybicolosin C from Lespedeza bicolor Attenuates Inflammation and Oxidative Stress via Nrf2/HO-1 and NF-κB/MAPK Pathways in Lipopolysaccharide-Induced Mouse Kupffer Cells
Abstract
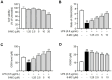
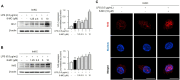
Lespedeza bicolor (L. bicolor) is known for its anti-inflammatory, antioxidant, and anticancer properties, making it a common choice in traditional medicine practices. Researchers in several recent studies have focused on isolating individual phytochemicals from this plant through chromatography analysis to explore their therapeutic potential. In our previous work, we identified 8-methoxybicolosin C (8-MC) as a novel flavonoid derivative, isolated and purified from the roots of L. bicolor, which exhibited inhibitory effects on cell proliferation. In this study, we further investigated the biological activities of 8-MC by examining its antioxidant and anti-inflammatory effects in LPS-induced mouse Kupffer cells. The results showed that 8-MC suppresses the expression of inflammation-related mediators, including inducible nitric oxide synthase (iNOS), nitric oxide (NO), and pro-inflammatory cytokines such as tumor necrosis factor (TNF)-α, interleukin (IL)-6, and IL-1β, in a dose-dependent manner. Additionally, 8-MC improves the GSH/GSSG balance by increasing glutathione (GSH) levels and decreasing oxidized glutathione (GSSG) levels. Interestingly, 8-MC was found to bind Keap1, preventing roteasomal degradation, and promoting the nuclear translocation of nuclear factor erythroid 2-related factor 2 (Nrf2), thereby increasing the expression of antioxidant-related proteins such as heme oxygenase-1 (HO-1). Moreover, 8-MC suppressed the activation of inflammatory signaling pathways, including c-Jun N-terminal kinases (JNKs) and p38 mitogen-activated protein kinases (MAPKs), while also inhibiting the nuclear translocation of nuclear factor kappa B (NF-κB), effectively reducing inflammatory responses. These findings collectively demonstrated that 8-MC possesses potent anti-inflammatory and antioxidant activities through the regulation of NF-κB, MAPK, and Nrf2/HO-1 signaling pathways. Consequently, 8-MC shows potential as a valuable therapeutic agent for managing various inflammatory disorders.
Keywords: 8-Methoxybicolosin C; MAPKs; NF-κB; Nrf2/HO-1 pathway; anti-inflammation; antioxidant.
Conflict of interest statement
The authors have no financial conflicts of interest to declare.
Figures






References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

