CYP2C19-Guided Voriconazole Therapy: A Precision Medicine Approach to Mitigate Adverse Effects in Japanese Patients
- PMID: 40825683
- PMCID: PMC12360865
- DOI: 10.1111/cts.70317
CYP2C19-Guided Voriconazole Therapy: A Precision Medicine Approach to Mitigate Adverse Effects in Japanese Patients
Abstract
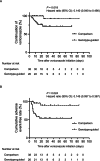
Voriconazole (VRCZ) is a triazole antifungal agent with a broad antifungal spectrum. It is metabolized by hepatic cytochrome P450 (CYP) isozyme CYP2C19, whose genetic polymorphism causes significant variability in drug efficacy and safety. Poor metabolizer alleles of CYP2C19 are more common in Asian populations, increasing the risk of supratherapeutic VRCZ levels. We have developed a novel nomogram based on CYP2C19 genetic polymorphisms. This study aimed to evaluate whether CYP2C19 genotype-guided VRCZ therapy reduces toxicity in Japanese patients. This retrospective study included 64 patients (genotype-guided group, n = 26; comparison group, n = 38). The primary outcome was defined as the composite incidence of adverse events commonly observed with VRCZ, represented by the combined occurrence of grade ≥ 2 hepatotoxicity and visual symptoms. Secondary outcomes included the proportion of patients maintaining VRCZ trough concentrations within the therapeutic range (1-4 μg/mL) and the treatment response at 28 days. The composite incidence of adverse events was significantly lower in the genotype-guided group than in the comparison group (p = 0.003). VRCZ discontinuation due to adverse events occurred in nine patients (23.7%) in the comparison group and one (3.8%) in the genotype-guided group (p = 0.039). More patients in the genotype-guided group were achieved through concentrations within the therapeutic range at the initial sampling point. However, treatment response rates did not differ significantly between the groups. VRCZ administration based on CYP2C19 genotyping improved therapeutic trough levels management and reduced adverse effects while maintaining therapeutic efficacy. These findings highlight the importance of CYP2C19 genotyping for VRCZ treatment in Japanese patients.
Keywords: CYP2C19; genotype‐guided; pharmacogenomics; precision medicine; therapeutic drug monitoring; voriconazole.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Ullmann A. J., Akova M., Herbrecht R., et al., “ESCMID* Guideline for the Diagnosis and Management of Candida Diseases 2012: Adults With Haematological Malignancies and After Haematopoietic Stem Cell Transplantation (HCT),” Clinical Microbiology and Infection 18, no. Suppl 7 (2012): 53–67, 10.1111/1469-0691.12041. - DOI - PubMed
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Medical

