Genetic isolation and metabolic complexity of an Antarctic subglacial microbiome
- PMID: 40825763
- PMCID: PMC12361377
- DOI: 10.1038/s41467-025-62753-3
Genetic isolation and metabolic complexity of an Antarctic subglacial microbiome
Abstract
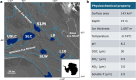
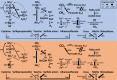
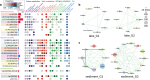
Microbes inhabiting and evolving in aquatic ecosystems beneath polar ice sheets subsist under energy-limited conditions while in relative isolation from surface gene pools and their common ancestral populations of origin. Samples obtained from beneath West Antarctic Ice Sheet (WAIS) allowed us to examine evolutionary relationships of and identify metabolic pathways in microbial genomes recovered from the Mercer Subglacial Lake (SLM) ecosystem. We obtained 1,374 single-cell amplified genomes (SAGs) from individual bacterial and archaeal cells that were isolated from samples of SLM's water column and sediments. These genomes reveal that a diversity of microorganisms including Patescibacteria exists in SLM. Comparative analyses show that most genomes correspond to new species and taxonomic groups, with phylogenomic and functional evidence supporting their genetic isolation from marine and surface biomes. Genomic data reveal diverse metabolisms in SLM that are capable of oxidizing organic and inorganic compounds via aerobic or anaerobic respiration. Distinct metabolic guild structures are observed for the subglacial populations, where trophic shifts from organotrophy to chemolithotrophy may depend on oxygen availability. Our SAG data suggest versatile metabolic capabilities in the characterized microbial assemblage, reveal key energy-generating strategies in the subglacial aquatic ecosystem, and provide a framework to assess microbial evolution beneath WAIS.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Horgan, H. J. et al. Subglacial Lake Whillans—Seismic observations of a shallow active reservoir beneath a West Antarctic ice stream. Earth Planet. Sci. Lett.331, 201–209 (2012).
-
- Fricker, H. A., Scambos, T., Bindschadler, R. & Padman, L. An active subglacial water system in West Antarctica mapped from space. Science315, 1544–1548 (2007). - PubMed
-
- Wadham, J. L. et al. The potential role of the Antarctic Ice Sheet in global biogeochemical cycles. Earth Environ. Sci. Trans. R. Soc. Edinb.104, 55–67 (2013).
-
- Venturelli, R. et al. Mid-Holocene grounding line retreat and readvance at Whillans Ice Stream, West Antarctica. Geophys. Res. Lett.47, e2020GL088476 (2020).
-
- Hodson, T. et al. Physical processes in Subglacial Lake Whillans, West Antarctica: inferences from sediment cores. Earth Planet. Sci. Lett.444, 56–63 (2016).
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

