Unveiling migraine subtype heterogeneity and risk loci: integrated genome-wide association study and single-cell transcriptomics discovery
- PMID: 40826382
- PMCID: PMC12360011
- DOI: 10.1186/s10194-025-02128-7
Unveiling migraine subtype heterogeneity and risk loci: integrated genome-wide association study and single-cell transcriptomics discovery
Abstract
Background: Migraine, a debilitating neurological disorder with distinct subtypes (migraine with aura [MA] and migraine without aura [MO]), exhibits genetic and spatial heterogeneity that remains poorly understood. While genetic correlations between subtypes are established, spatially resolved molecular mechanisms driving their divergent clinical phenotypes-particularly in tissue microenvironments-are unclear, limiting targeted therapeutic development.
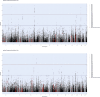
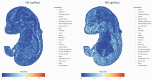
Methods: We integrated genome-wide association study (GWAS) data from FinnGen R11 and international cohorts with transcriptomic, epigenomic, and spatially resolved single-cell spatial transcriptomics (sc-ST) profiles. Genetic correlations and functional annotations were assessed using Linkage Disequilibrium Score Regression (LDSC), High-Definition Likelihood (HDL), and partitioned heritability analyses. A multi-omics framework combined Summary Mendelian Randomization (SMR) for expression and methylation quantitative trait loci (eQTL/mQTL), Functional Summary-based Imputation (FUSION), Multi-marker Analysis of GenoMic Annotation (MAGMA), Joint-Tissue Imputation Enhanced PrediXcan Analysis (JTI-PrediXcan), and the Polygenic Priority Score (PoPS) to systematically prioritize genes based on methodological robustness (≥ 2 analytical approaches) and cross-subtype consistency. Tissue-enriched specificity was validated via genetically informed spatial mapping of cells for complex traits (gsMap), a novel algorithm integrating sc-ST and GWAS data to map subtype-associated cellular architectures at single-cell resolution across embryonic tissues.
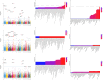
Results: LDSC and HDL confirmed strong genetic correlations between MA and MO. But they showed divergent functional architectures in functional genomic annotations, with MA enriched in conserved regulatory elements (e.g., Backgrd_Selection_StatL2_0, enrichment = 1.38, P = 5.47 × 10-6) and MO in vascular pathways (e.g., GERP.NSL2_0, enrichment = 2.12, P = 1.04 × 10-6). Sc-ST revealed spatially divergent niches: MA showed prenatal enrichment in neural crest-derived tissues (jaw primordium, p = 0.0039) and hypothalamic microglial adjacencies, aligning with neuroimmune regulation, while MO exhibited peripheral tropism in vascular smooth muscle and gut-brain interfaces, corroborated by LDSC-SEG/MAGMA vascular pathways. Multi-omics integration identified high-confidence cross-subtype genes (LRP1 [PoPS: Overall = 3.67, MO = 0.80], PHACTR1 [PoPS: Overall = 2.65, MA = 0.33, MO = 1.28], STAT6 [PoPS: Overall = 3.00, MO = 2.29], RDH16, TTC24, ZBTB39, FHL5, MEF2D, NAB2, UFL1, and REEP3) supported by ≥ 2 methods. Subtype-specific genes included MA-associated neuronal regulators (CACNA1A, KLHDC8B) and MO-specific vascular/metabolic genes (e.g., ACO2, BCAR1, CCDC134).
Conclusion: Our study delineates spatially constrained mechanisms underlying migraine heterogeneity: MA arises from neuroimmune-epigenetic dysregulation, while MO is driven by vascular-metabolic perturbations. Key genes and pathways provide actionable targets for subtype-specific therapies. By bridging genetic architecture with spatial biology, we redefine migraine pathogenesis and precision intervention strategies.
Keywords: Migraine subtypes; Multi-omics integration; Precision medicine; Single-cell spatial transcriptomics; Therapeutic targets.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The dataset used in this study is publicly available, and ethical approval and informed consent were obtained before implementation. Therefore, our study requires no additional informed consent or ethical approval. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Headache Classification Committee of the International Headache Society (IHS) (2018) The international classification of headache disorders, 3rd edition. Cephalalgia 38(1):1–211 - PubMed
-
- Charles A (2018) The pathophysiology of migraine: implications for clinical management. Lancet Neurol 17(2):174–182 - PubMed
-
- Leao AA (1947) Further observations on the spreading depression of activity in the cerebral cortex. J Neurophysiol 10(6):409–414 - PubMed
-
- Karatas H, Erdener SE, Gursoy-Ozdemir Y, Lule S, Eren-Kocak E, Sen ZD et al (2013) Spreading depression triggers headache by activating neuronal Panx1 channels. Science 339(6123):1092–1095 - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

