The dual GLP-1 and GIP receptor agonist tirzapetide provides an unintended interaction with the β-adrenoceptors and plays a role in glucose metabolism in hyperglycemic or senescent cardiac cells
- PMID: 40826463
- PMCID: PMC12362930
- DOI: 10.1186/s12933-025-02828-z
The dual GLP-1 and GIP receptor agonist tirzapetide provides an unintended interaction with the β-adrenoceptors and plays a role in glucose metabolism in hyperglycemic or senescent cardiac cells
Abstract
Background: A dual glucose-dependent insulinotropic polypeptide (GIP) and glucagon-like peptide-1 (GLP1) receptor agonist, tirzepatide (TZPD), is a novel cardioprotective agent, particularly in metabolic disturbances-related co-morbidities, however, there is no exact study to emphasize its possible unintended action in cardiac cells.
Objective: Considering a relationship between the trafficking of incretin receptors in a manner not anticipated by the standard way of cAMP as a primary actor in TZPD action, together with the role of cAMP depression in cardiac dysfunction, here, we aimed to elucidate a pattern of unintended receptor interactions of TZPD and molecular processes underlying the pleiotropic effects of TZPD through modulation of the β-adrenoceptors (β-ARs) signaling in cardiomyocytes.
Methods: To establish the multifaceted cardioprotective function and underlying mechanisms of TZPD against hyperglycemia (HG)-or senescence (SC)-induced cardiac dysfunction, H9c2 cells were treated with and without TZPD. We also used β3-ARs overexpressed H9c2 cells (β3OE) for comparisons.
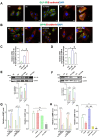
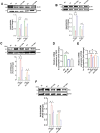
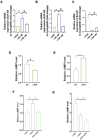
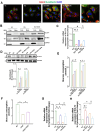
Results: The TZPD intervention ameliorated the HG or SC phenotypes in the cardiac cells via alleviation in protein levels of GLP-1R and GIP-R as well as production of cAMP or cGMP, even in the presence of these receptor antagonisms. TZPD also increased the levels of β1- and β2-ARs while significantly decreasing activated β3-ARs and PKG, being parallel to normalizations in the cAMP and cGMP in the presence of the antagonisms of these receptors. The therapeutic effects of TZPD on similar parameters of the β3OE group of cells can strongly verify its unintended action among multifaceted effects in either HG or SC cells. In addition, molecular dynamics simulations indicated that TZPD binds with the highest affinity to GLP-1R and β3-ARs rather than GIP-R and then relatively lower but almost similar affinities to β1- and β2-ARs. Furthermore, mechanistically, the cardioprotective effect of TZPD includes significant regulation of the cellular Ca2+, at most, modulating the proteins in β-ARs signaling pathways. Moreover, TZPD could significantly increase not only the depressed protein level but also the translocation of GLUT4 on the sarcolemma, promoting glucose uptake in the HG or SC groups independent of its receptor actions.
Conclusions: Our findings indicate that TZPD, with its multifaceted role, has beneficial effects on cardiac cells by positively modulating β-ARs signaling and glucose metabolism rather than on-target receptor action. Furthermore, we demonstrated how TZPD can engage the different targets with distinct signaling motifs at the sarcolemma.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

