Real-World Multinational Survey of Chronic Inflammatory Demyelinating Polyneuropathy: Disease Characteristics and Therapeutic Landscape
- PMID: 40826893
- PMCID: PMC12361836
- DOI: 10.1111/jns.70047
Real-World Multinational Survey of Chronic Inflammatory Demyelinating Polyneuropathy: Disease Characteristics and Therapeutic Landscape
Abstract
Background and aims: Chronic inflammatory demyelinating polyneuropathy (CIDP) is an immune-mediated syndrome characterized by progressive muscle weakness and sensory impairment. Clinical similarities with other neuropathies can cause misdiagnoses and delayed diagnoses. Additionally, a large proportion of patients appropriately treated according to current guidelines still show residual disability. This real-world study aimed to characterize a global cohort of patients with CIDP.
Methods: Data were drawn from the Adelphi CIDP Disease Specific Programme, a cross-sectional survey with retrospective data collection, conducted in China, France, Germany, Italy, Japan, Spain, the United Kingdom, and the United States between September 2022 and April 2023. Neurologists and neuromuscular specialists reported on patient demographic and clinical characteristics at the time of the survey. Patients self-reported treatment satisfaction, disease control, and health-related outcome measures.
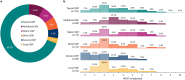
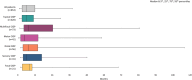
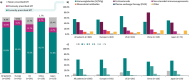
Results: Overall, 164 physicians provided data for 1056 patients, with 428 (40.5%) providing self-reported data. Patients were diagnosed with typical CIDP (69.2%) and variant CIDP (30.8%). Overall, initial misdiagnosis occurred in 37.2% of patients, with a median (interquartile range) diagnostic delay of 6.0 (3.0-12.0) months. Maintenance therapy was prescribed for 81.6% of patients, with corticosteroid use ranging from 25.7% in the United States to 80.0% in China. Some patients were dissatisfied by treatment outcomes (11.0%) and symptom control (12.2%). Overall, mean (SD) patient-reported scores were 62.1 (20.4) for I-RODS, 35.0 (11.1) for FACIT fatigue, and 0.662 (0.253) for EQ-5D-5L.
Interpretation: Diagnostic delay and misdiagnoses were common occurrences across typical CIDP and variant CIDP. Despite the use of guideline treatments, there were unmet needs and a continued disease burden for patients.
Keywords: chronic inflammatory demyelinating polyneuropathy; disease burden; healthcare resource utilization; patient reported outcomes; treatment patterns.
© 2025 The Author(s). Journal of the Peripheral Nervous System published by Wiley Periodicals LLC on behalf of Peripheral Nerve Society.
Conflict of interest statement
Luis Querol is an employee of Hospital de la Santa Creu i Sant Pau. Luis Querol received research grants from Instituto de Salud Carlos III—Ministry of Economy and Innovation (Spain), CIBERER, Fundació La Marató, GBS‐CIDP Foundation International, and ArgenX. Luis Querol received speaker or expert testimony honoraria from CSL Behring, Novartis, Sanofi‐Genzyme, Merck, Annexon, Alnylam, ArgenX, Dianthus, LFB, Avilar Therapeutics, Lycia Therapeutics, Nuvig Therapeutics, Takeda, and Roche. Luis Querol serves at Clinical Trial Steering Committees for Sanofi Genzyme, ArgenX, and Takeda and is Principal Investigator for UCB's CIDP01 trial and Sanofi's Mobilize and Vitalize trials.
Simon Rinaldi is an employee of Nuffield Department of Clinical Neurosciences, University of Oxford. He is currently supported by grants from the Medical Research Council (UK), GBS|CIDP Foundation International, and British Medical Association. He has previously been supported by grants from the Medical Research Council (UK), Wellcome Trust, National Institute of Health Research (NIHR), the Pathological Society of Great Britain and Ireland, and the University of Oxford's John Fell Fund. He has received honoraria for lectures given at the request of Excemed, Fresenius, CSL Behring, UCB, argenx, the Beijing Association of Holistic and Integrated Medicine, and the Irish Institute of Clinical Neuroscience. He has been a paid consultant for argenx, Annexon, Dianthus, Takeda, and Hansa Biopharma. He is an unpaid member of the medical advisory board of the Guillain‐Barré syndrome and Related Inflammatory Neuropathies (GAIN) charity and of the Inflammatory Neuropathy Consortium (INC) board. He has previously received reduced registration fees, travel grants, and scientific prizes from the Peripheral Nerve Society. He runs a not‐for‐profit diagnostic testing service for nodal/paranodal antibodies.
Gerd Meyer zu Hörste is an employee of University Hospital Münster. In the last 5 years, GMzH received speaker honoraria from Alexion, LFB Pharma, Amgen, Argenx; compensation for serving on advisory boards for LFB Pharma, Immunovant, Roche; and received project‐related research funding from Merck (Germany), Biogen, Roche. He received no compensation for his activity in this study.
Andras Borsi, Charlotte Gary, Wim Noel, Wisam Karmous, and Giorgio Maria Boggia are employees of Johnson & Johnson Innovative Medicine and may hold stock.
Jonathan DeCourcy, Yasmin Taylor, and Jack Wright are employees of Adelphi Real World.
Figures




References
-
- Dziadkowiak E., Waliszewska‐Prosol M., Nowakowska‐Kotas M., Budrewicz S., Koszewicz Z., and Koszewicz M., “Pathophysiology of the Different Clinical Phenotypes of Chronic Inflammatory Demyelinating Polyradiculoneuropathy (CIDP),” International Journal of Molecular Sciences 23, no. 1 (2021): 179, 10.3390/ijms23010179. - DOI - PMC - PubMed
-
- Van den Bergh P. Y. K., van Doorn P. A., Hadden R. D. M., et al., “European Academy of Neurology/Peripheral Nerve Society Guideline on Diagnosis and Treatment of Chronic Inflammatory Demyelinating Polyradiculoneuropathy: Report of a Joint Task Force‐Second Revision,” European Journal of Neurology 28, no. 11 (2021): 3556–3583, 10.1111/ene.14959. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

