Early Pseudoprogression After Tarlatamab in Small-Cell Lung Cancer: A Case Report
- PMID: 40827251
- PMCID: PMC12358216
- DOI: 10.1002/rcr2.70319
Early Pseudoprogression After Tarlatamab in Small-Cell Lung Cancer: A Case Report
Abstract
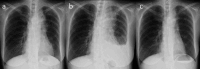
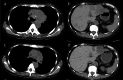
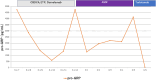
Pseudoprogression, a transient radiographic flare caused by immune infiltration, is common after immune-checkpoint inhibitors but has not been reported with tarlatamab, a bispecific T-cell engager approved for third-line small-cell lung cancer (SCLC). A 57-year-old woman with extensive-stage SCLC and syndrome of inappropriate antidiuretic hormone secretion (SIADH) received tarlatamab. Within hours, she developed bone pain; Day 7 imaging showed marked tumour swelling and pleural effusion despite negative cytology and rising serum sodium. Therapy continued. By Day 13, computed tomography demonstrated regression of thoracic and hepatic lesions and falling pro-gastrin-releasing peptide (pro-GRP). Early pseudoprogression and paraneoplastic biomarker improvement may predict efficacy.
Keywords: bispecific T‐cell engager therapy; pseudoprogression; small‐cell lung cancer; tarlatamab.
© 2025 The Author(s). Respirology Case Reports published by John Wiley & Sons Australia, Ltd on behalf of The Asian Pacific Society of Respirology.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures
References
-
- Chen M. Y. and Zeng Y. C., “Pseudoprogression in Lung Cancer Patients Treated With Immunotherapy,” Critical Reviews in Oncology/Hematology 169 (2022): 103531. - PubMed
-
- Imataki O., Uemura M., Fujita H., and Kadowaki N., “Pathological Landscape of Tumor Flare Reaction to Epcoritamab Treatment,” International Journal of Hematology 120, no. 4 (2024): 467–471. - PubMed
-
- Ahn M. J., Cho B. C., Felip E., et al., “Tarlatamab for Patients With Previously Treated Small‐Cell Lung Cancer,” New England Journal of Medicine 389, no. 22 (2023): 2063–2075. - PubMed
LinkOut - more resources
Full Text Sources

