Mechanism of Lipi Jiangzhuo Decoction in Improving Metabolic Dysfunction-Associated Steatohepatitis Through the PERK/PINK1/GPx4 Pathway
- PMID: 40827263
- PMCID: PMC12358153
- DOI: 10.2147/JIR.S532630
Mechanism of Lipi Jiangzhuo Decoction in Improving Metabolic Dysfunction-Associated Steatohepatitis Through the PERK/PINK1/GPx4 Pathway
Abstract
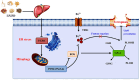
Background: Metabolic dysfunction-associated steatohepatitis (MASH) is characterized primarily by hepatocyte lipoapoptosis and hepatic inflammation, frequently developing from overweight/obesity. To date, no specific therapeutics exist to reverse MASH. Although resmetirom has been approved in some regions, patients in many Asian countries, including China, still lack access to approved pharmacotherapy for MASH. Lipi Jiangzhuo decoction (LPJZD) is a promising traditional Chinese medicine formula for MASH. However, to date, there have been no comprehensive studies clarifying its potential mechanism of action. This study aims to elucidate the underlying mechanism of action of LPJZD in the treatment of MASH.
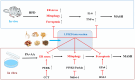
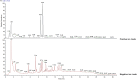
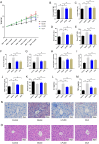
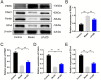
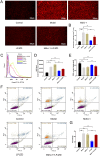
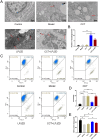
Materials and methods: A MASH mouse model was established by feeding a high-fat diet and subjecting them to fatigue protocols and cold stress for 12 weeks. After treating MASH mice with LPJZD, biochemical assays were conducted to assess the efficacy of LPJZD in alleviating the MASH symptoms. In addition, the in vitro effects of LPJZD on MASH were evaluated using L-02 cells. Specifically, we analyzed the effect of LPJZD on endoplasmic reticulum (ER) stress, mitophagy, and ferroptosis by Western blot analysis, flow cytometry, immunofluorescence staining, and enzyme-linked immunosorbent assay.
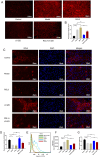
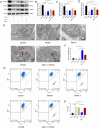
Results: In vivo, LPJZD effectively improved the inflammatory response, reduced body weight and blood lipid levels, improved liver function, reduced liver lipid droplet accumulation, and ameliorated the pathological status of MASH mice. In vitro, LPJZD effectively inhibited ferroptosis by reducing ferrous ions and reactive oxygen species levels, increasing GPx4 protein expression, elevating glutathione levels, and ameliorating mitochondrial swelling and matrix thinning. Simultaneously, LPJZD activated mitophagy by increasing PINK1 and Parkin protein expression, augmenting mitophagosome number, and restoring mitochondrial membrane potential. Additionally, LPJZD suppressed ER stress by decreasing PERK protein expression. Notably, activation of ER stress using a PERK activator attenuated LPJZD's effects on mitophagy activation and ferroptosis inhibition, inhibition of mitophagy via a PINK1 inhibitor diminished LPJZD's anti-ferroptotic effect, and administration of a GPx4 inhibitor reduced LPJZD's suppression of ferroptosis. Therefore, these results demonstrate that LPJZD ameliorates MASH by regulating the PERK/PINK1/GPx4 pathway.
Conclusion: LPJZD can improve MASH by regulating ER stress-mitophagy -ferroptosis axis in liver cells. The role of LPJZD in anti-inflammatory therapy provides new insights for the clinical prevention and treatment of MASH.
Keywords: Lipi Jiangzhuo decoction; endoplasmic reticulum stress; ferroptosis; metabolic dysfunction-associated steatohepatitis; mitophagy.
© 2025 Pan et al.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



Similar articles
-
Prescription of Controlled Substances: Benefits and Risks.2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. 2025 Jul 6. In: StatPearls [Internet]. Treasure Island (FL): StatPearls Publishing; 2025 Jan–. PMID: 30726003 Free Books & Documents.
-
Injinoryeong-San attenuates metabolic dysfunction-associated steatohepatitis via regulation of YAP/TAZ-signaling pathway.J Ethnopharmacol. 2025 Jul 17;353(Pt A):120292. doi: 10.1016/j.jep.2025.120292. Online ahead of print. J Ethnopharmacol. 2025. PMID: 40683423
-
Therapeutic effects of adipose tissue-derived mesenchymal stem cells on ER stress in a murine model of metabolic dysfunction-associated steatohepatitis: an in vivo and in vitro study.Stem Cell Res Ther. 2025 Jul 6;16(1):349. doi: 10.1186/s13287-025-04482-4. Stem Cell Res Ther. 2025. PMID: 40619419 Free PMC article.
-
The Black Book of Psychotropic Dosing and Monitoring.Psychopharmacol Bull. 2024 Jul 8;54(3):8-59. Psychopharmacol Bull. 2024. PMID: 38993656 Free PMC article. Review.
-
Cost-effectiveness of using prognostic information to select women with breast cancer for adjuvant systemic therapy.Health Technol Assess. 2006 Sep;10(34):iii-iv, ix-xi, 1-204. doi: 10.3310/hta10340. Health Technol Assess. 2006. PMID: 16959170
References
LinkOut - more resources
Full Text Sources

