Patient and First-Degree Relatives Perceptions About Prediction and Prevention of Inflammatory Bowel Disease-A Multinational Survey
- PMID: 40827758
- PMCID: PMC12463702
- DOI: 10.1002/ueg2.70091
Patient and First-Degree Relatives Perceptions About Prediction and Prevention of Inflammatory Bowel Disease-A Multinational Survey
Abstract
Background and objective: While significant advances have been made in identifying biomarkers for predicting inflammatory bowel disease (IBD) onset, little is known about the willingness of at-risk individuals to undergo predictive testing and preventive interventions. This study aimed to assess acceptance of predictive tests and preventive interventions among individuals at risk of inflammatory bowel disease and identify factors influencing their decisions.
Methods: An anonymized electronic survey was distributed to parents of children at risk of inflammatory bowel disease and first-degree relatives (FDRs) of IBD patients via clinicians and patient associations. The survey assessed acceptance of predictive tests, preventive interventions, and influencing variables.
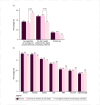
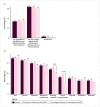
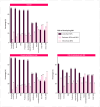
Results: A total of 1327 participants (74% women, mean age 42 ± 18 years) from 66 countries responded to the survey. Of these, 88% were parents of children at risk, and 12% were FDRs. Eighty-five percent were willing to embark in predictive testing, preferring blood analysis (91%), stool tests (89%), saliva tests (78%) or intestinal ultrasound (67%). Lower perceived IBD impact and higher disease knowledge reduced the odds of test acceptance. Preventive interventions were accepted by 98%, with dietary changes (85%), physical exercise (81%), and probiotics (71%) being the preferred. Acceptance of oral (38%) or intravenous/subcutaneous immunosuppressive treatments (32%) depended on their efficacy, difficulty, and risks.
Conclusion: Most respondents preferred minimally invasive predictive tests and non-pharmacological preventive measures, although more than one-third would be willing to undergo immunosuppressive medications to prevent disease onset. Disease knowledge and quality-of-life perceptions influenced preferences. This data provides important information for the development of IBD prediction and prevention strategies.
Keywords: Crohn's disease; first‐degree relatives; inflammatory bowel disease; prediction; prevention; relatives; risk; survey; ulcerative colitis.
© 2025 The Author(s). United European Gastroenterology Journal published by Wiley Periodicals LLC on behalf of United European Gastroenterology.
Conflict of interest statement
M.J. has received research grants for other investigator‐driven studies from Takeda, and the NOVO Nordisk Foundation (grant no. NNF23OC0081717); consulting/advisory board fees from Ferring, Takeda, AbbVie, PharmaCosmos and Tillots Pharma; speaker’s fees from Tillotts Pharma, MSD, Ferring, and Takeda. I.R.‐L. has received consulting/advisory board fees from Adacyte, Pfizer, Abbvie, Janssen, Celltrion, and Alfasigma, and research support from AbbVie. P.J. has served as speaker, consultant, and advisory member for AbbVie, Amgen, Aspen Pharmacare, Bristol Myers Squibb, CLS Vifor Pharma, Eli Lilly, Ferring Pharmaceuticals, Gilead Sciences, Johnson & Johnson, Merck Sharp & Dohme, Pfizer, Pierre Fabre, Sandoz, Takeda, Tillotts Pharma AG, UCB Pharma. C.S. is the co‐founder of MiTest Health LLC (software company), licensed to Takeda. J.T. has received consulting/advisory board fees from Abbvie, Pfizer, Janssen, Tillots, Sandoz, and Lilly; grant support from Janssen and Abbvie. The other authors have no conflicts of interest to declare. INTERCEPT project is funded by the European Union, the private members, and those contributing partners of the IHI JU. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the aforementioned parties. Neither of the aforementioned parties can be held responsible for them.
Figures

References
-
- Machold K. P., Landewé R., Smolen J. S., et al., “The Stop Arthritis Very Early (SAVE) Trial, an International Multicentre, Randomised, Double‐Blind, Placebo‐Controlled Trial on Glucocorticoids in Very Early Arthritis,” Annals of the Rheumatic Diseases 69, no. 3 (March 2010): 495–502, 10.1136/ard.2009.122473. - DOI - PubMed
-
- Verstappen S. M. M., McCoy M. J., Roberts C., Dale N. E., Hassell A. B., and Symmons D. P. M., “Beneficial Effects of a 3‐Week Course of Intramuscular Glucocorticoid Injections in Patients With Very Early Inflammatory Polyarthritis: Results of the STIVEA Trial,” Annals of the Rheumatic Diseases 69, no. 3 (March 2010): 503–509, 10.1136/ard.2009.119149. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

