Human IgE monoclonal antibodies define two unusual epitopes trapping dog allergen Can f 1 in different conformations
- PMID: 40828364
- PMCID: PMC12363406
- DOI: 10.1002/pro.70269
Human IgE monoclonal antibodies define two unusual epitopes trapping dog allergen Can f 1 in different conformations
Abstract
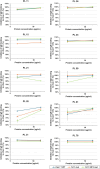
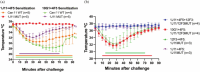
Molecular analysis of interactions between IgE antibody and allergen allows the structural basis of IgE recognition to be defined. Human IgE (hIgE) epitopes of respiratory lipocalin allergens, including Can f 1, remain elusive due to a lack of IgE-allergen complexes. This study aims to map the structure of allergenic epitopes on Can f 1. The fragment antigen-binding (Fab) regions of Can f 1 specific human IgE monoclonal antibodies (hIgE mAb) were used to determine the structures of IgE epitopes. Epitope mutants were designed to target Can f 1 epitopes. Immunoassays and a human FcεRIα transgenic mouse model of passive anaphylaxis in vivo were used to assess the functional activity of epitope mutants. Crystal structures of natural or recombinant Can f 1 complexed with two hIgE mAb 1J11 and 12F3 Fabs, respectively, were determined. The hIgE mAb bound to two partially overlapping epitopes and recognized two different Can f 1 conformations. The hIgE mAb 12F3 showed an unusual mode of binding by protruding its heavy chain CDR3 inside the Can f 1 calyx. Epitope mutants generated based on the structural analyses displayed a 64%-89% reduction in IgE antibody binding and failed to induce passive anaphylaxis in a human FcεRIα transgenic mouse model. In summary, the structures of Can f 1-hIgE Fab complexes revealed two unique and partially overlapping epitopes on Can f 1. The modification of the identified IgE epitopes provides a pathway for the design of hypoallergens to treat dog allergies.
Keywords: Can f 1; IgE/allergen complexes; conformational epitope; dog allergen; epitope mutants; human IgE antibody; lipocalins.
© 2025 The Author(s). Protein Science published by Wiley Periodicals LLC on behalf of The Protein Society.
Figures



Similar articles
-
Structural analysis of human IgE monoclonal antibody epitopes on dust mite allergen Der p 2.J Allergy Clin Immunol. 2024 Aug;154(2):447-457. doi: 10.1016/j.jaci.2024.04.017. Epub 2024 Apr 30. J Allergy Clin Immunol. 2024. PMID: 38697404 Free PMC article.
-
Pre-clinical allergenicity assessment of IgE epitope-targeted Der p 2 mutants demonstrate potential as hypoallergenic AIT candidates.Front Immunol. 2025 Jun 27;16:1623920. doi: 10.3389/fimmu.2025.1623920. eCollection 2025. Front Immunol. 2025. PMID: 40655157 Free PMC article.
-
Interaction between expression of CD23 on B-lymphocytes and level of specific IgE against molecular components of NPC2 family, lipocalins, uteroglobins, and molecular components of molds and yeast.J Immunotoxicol. 2025 Dec;22(1):2531794. doi: 10.1080/1547691X.2025.2531794. Epub 2025 Aug 11. J Immunotoxicol. 2025. PMID: 40785403
-
ImmunoCAP® ISAC and Microtest for multiplex allergen testing in people with difficult to manage allergic disease: a systematic review and cost analysis.Health Technol Assess. 2016 Sep;20(67):1-178. doi: 10.3310/hta20670. Health Technol Assess. 2016. PMID: 27623692 Free PMC article.
-
Epitope-Specific Antibodies in Allergic Disease and Clinical Tolerance.Immunol Rev. 2025 Jul;332(1):e70042. doi: 10.1111/imr.70042. Immunol Rev. 2025. PMID: 40542457 Review.
References
-
- Berger C, Weber‐Bornhauser S, Eggenberger J, Hanes J, Plückthun A, Bosshard HR. Antigen recognition by conformational selection. FEBS Lett. 1999;450(1–2):149–153. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

