In vivo characterization of Achilles subtendon function and morphology within the tendon cross section and along the free tendon
- PMID: 40828574
- PMCID: PMC12427142
- DOI: 10.1152/japplphysiol.00479.2025
In vivo characterization of Achilles subtendon function and morphology within the tendon cross section and along the free tendon
Abstract
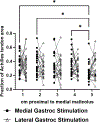
The Achilles tendon is composed of three distinct fascicle bundles, or subtendons, each originating from the head of one of the three triceps surae muscles. In a healthy tendon, these subtendons slide relative to each other during muscle contractions. This subtendon sliding is reduced in older adults and individuals who suffer from an Achilles tendon injury. However, subtendon sliding is challenging to quantify in low-load scenarios that are critical for monitoring subtendon biomechanics in patients with mechanically compromised tendons, such as following an Achilles tendon rupture and repair. The purpose of this study was to develop a reliable method to characterize subtendon behavior in vivo using combined transverse plane ultrasound imaging and neuromuscular electrical stimulation of individual gastrocnemii. We used a Kanade-Lucas-Tomasi point tracking algorithm to quantify tendon displacement during isolated muscle stimulations. Next, we applied k-means clustering to characterize heterogeneous subtendon behavior within the tendon cross section. The tendon cross section displayed differential displacement patterns depending on the stimulated muscle (P < 0.0001), and these displacements differed along the tendon length during lateral gastrocnemius stimulations (P = 0.004). These results reflect possible differences in load-sharing between adjacent subtendons and differing muscle-tendon dynamics among the triceps surae muscles. Finally, this method confirmed no bilateral differences in subtendon behavior and demonstrated high intersession reliability (intraclass correlation > 0.74). Overall, this study furthers our understanding of the differential muscle-tendon dynamics of individual Achilles subtendons within the tendon cross section and along the tendon length.NEW & NOTEWORTHY Achilles subtendon function and morphology are challenging to characterize in vivo. This study used transverse plane ultrasound imaging and neuromuscular electrical stimulation to characterize the behavior of individual subtendons both within the tendon cross section and along the free tendon. It is the first study to demonstrate functional behavior of the Achilles subtendons using these combined tools. In addition, the lack of bilateral differences in healthy individuals presents this tool's potential to quantify altered subtendon function post injury.
Keywords: Achilles subtendons; muscular stimulation; ultrasound.
Figures





Update of
-
In vivo characterization of Achilles subtendon function and morphology within the tendon cross section and along the free tendon.bioRxiv [Preprint]. 2025 Jun 1:2025.05.29.656873. doi: 10.1101/2025.05.29.656873. bioRxiv. 2025. Update in: J Appl Physiol (1985). 2025 Sep 1;139(3):812-822. doi: 10.1152/japplphysiol.00479.2025. PMID: 40661625 Free PMC article. Updated. Preprint.
References
-
- Edama M, Kubo M, Onishi H, Takabayashi T, Inai T, Yokoyama E, et al. The twisted structure of the human Achilles tendon. Scand J Med Sci Sports. 2015. Oct 1;25(5):e497–503. - PubMed
-
- Pękala PA, Henry BM, Ochała A, Kopacz P, Tatoń G, Młyniec A, et al. The twisted structure of the Achilles tendon unraveled: A detailed quantitative and qualitative anatomical investigation. Scand J Med Sci Sports. 2017. Dec 1;27(12):1705–15. - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

