Loss of tumor cell MHC class II drives MAPK inhibitor insensitivity of BRAF-mutant anaplastic thyroid cancers
- PMID: 40828595
- PMCID: PMC12520682
- DOI: 10.1172/JCI191781
Loss of tumor cell MHC class II drives MAPK inhibitor insensitivity of BRAF-mutant anaplastic thyroid cancers
Abstract
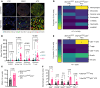
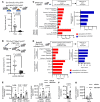
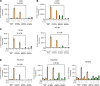
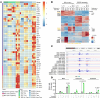
Cancer cells present neoantigens dominantly through MHC class I (MHCI) to drive tumor rejection through cytotoxic CD8+ T cells. There is growing recognition that a subset of tumors express MHC class II (MHCII), causing recognition of antigens by TCRs of CD4+ T cells that contribute to the antitumor response. We found that mouse BrafV600E-driven anaplastic thyroid cancers (ATCs) responded markedly to the RAF plus MEK inhibitors dabrafenib and trametinib (dab/tram) and that this was associated with upregulation of MhcII in cancer cells and increased CD4+ T cell infiltration. A subset of recurrent tumors lost MhcII expression due to silencing of Ciita, the master transcriptional regulator of MhcII, despite preserved IFN-γ signal transduction, which could be rescued by EZH2 inhibition. Orthotopically implanted Ciita-/- and H2-Ab1-/- ATC cells into immune-competent mice became unresponsive to the MAPK inhibitors. Moreover, depletion of CD4+, but not CD8+, T cells also abrogated the response to dab/tram. These findings implicate MHCII-driven CD4+ T cell activation as a key determinant of the response of Braf-mutant ATCs to MAPK inhibition.
Keywords: Antigen; Cancer; Endocrinology; Immunology; Thyroid disease.
Conflict of interest statement
Figures


Update of
-
Loss of tumor cell MHC Class II drives insensitivity of BRAF-mutant anaplastic thyroid cancers to MAPK inhibitors.bioRxiv [Preprint]. 2025 Jan 29:2025.01.27.635086. doi: 10.1101/2025.01.27.635086. bioRxiv. 2025. Update in: J Clin Invest. 2025 Aug 19;135(20):e191781. doi: 10.1172/JCI191781. PMID: 40093098 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

