Hypertension promotes bone loss and fragility by favoring bone resorption in mouse models
- PMID: 40828611
- PMCID: PMC12547992
- DOI: 10.1172/JCI184325
Hypertension promotes bone loss and fragility by favoring bone resorption in mouse models
Abstract
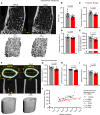
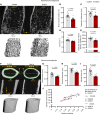
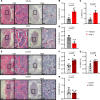
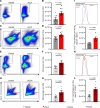
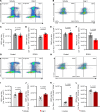
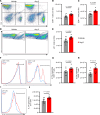
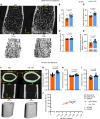
Inflammatory diseases contribute to secondary osteoporosis. Hypertension is a highly prevalent inflammatory condition that is clinically associated with reduced bone mineral density and increased risk of fragility fracture. In this study, we showed that a significant loss in bone mass and strength occurs in two preclinical models of hypertension. This accompanied increases in immune cell populations, including monocytes, macrophages, and IL-17A-producing T cell subtypes in the bone marrow of hypertensive mice. Neutralizing IL-17A in angiotensin II-infused mice blunted hypertension-induced loss of bone mass and strength as a result of decreased osteoclastogenesis. Likewise, the inhibition of the CSF1 receptor blunted loss of bone mass and prevented loss of bone strength in hypertensive mice. In an analysis of UK Biobank data, circulating bone remodeling markers exhibited striking associations with blood pressure and bone mineral density in more than 27,000 humans. These findings illustrate a potential mechanism by which hypertension activates immune cells in the bone marrow, encouraging osteoclastogenesis and eventual loss in bone mass and strength.
Keywords: Bone biology; Bone disease; Bone marrow; Hypertension; Immunology; Inflammation.
Conflict of interest statement
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

